
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-11-2020
Date: 10-8-2016
Date: 13-6-2019
|
Avogadro’s Law: The amount of gas
Amedeo Avogadro determined, from his study of gases, that equal volumes of gases at the same temperature and pressure contain equal numbers of gas particles. So Avogadro’s law says that the volume of a gas is directly proportional to the number of moles of gas (number of gas particles) at a constant temperature and pressure. Mathematically, Avogadro’s law looks like this: V = kn (at constant temperature and pressure) In this equation, k is a constant and n is the number of moles of gas. If you have a number of moles of gas (n1) at one volume (V1), and the moles change due to a reaction (n2), the volume also changes (V2), giving you the equation
V1/n1 = V2/n2
A very useful consequence of Avogadro’s Law is that you can calculate the volume of a mole of gas at any temperature and pressure. An extremely useful form to know when calculating the volume of a mole of gas is that 1 mole of any gas at STP occupies 22.4 liters. STP in this case is not an oil or gas additive; it stands for standard temperature and pressure.
✓ Standard pressure: 1.00 atm (760 torr or mm Hg)
✓ Standard temperature: 273 K
This relationship between moles of gas and liters gives you a way to convert the gas from a mass to a volume. For example, suppose that you have 50.0 grams of oxygen gas (O2) and you want to know its volume at STP. You can set up the problem like this
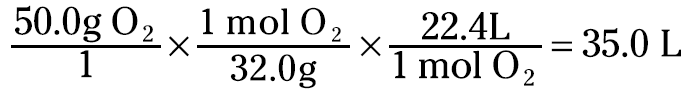
You now know that the 50.0 grams of oxygen gas occupies a volume of 35.0 liters at STP.
If the gas isn’t at STP, you can use the combined gas law (from the preceding section) to find the volume at the new pressure and temperature — or you can use the ideal gas equation, which I show you next.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|