
Limiting reactants
 المؤلف:
John T. Moore, EdD
المؤلف:
John T. Moore, EdD
 المصدر:
Chemistry Essentials For Dummies
المصدر:
Chemistry Essentials For Dummies
 الجزء والصفحة:
p 133
الجزء والصفحة:
p 133
 24-1-2017
24-1-2017
 6030
6030
Limiting reactants
In a chemical reaction, you normally run out of one of the reactants and have some others left over. The reactant you run out of first is called the limiting reactant, and it determines the amount of product formed. (In some of the problems sprinkled throughout this chapter, I tell you which reactant is the limiting one by saying you have an excess of the other reactant(s).) In this section, I show you how to calculate which reactant is the limiting reactant. Here is a reaction between ammonia and oxygen.
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
Suppose that you start out with 100.0 grams each of both ammonia and oxygen, and you want to know how many grams of NO (nitrogen monoxide, sometimes called nitric oxide) you can produce. You must determine the limiting reactant and base your stoichiometric calculations on it.
To figure out which reactant is the limiting reactant, you calculate the mole-to-coefficient ratio: Calculate the number of moles of both ammonia and oxygen, and then divide each by their coefficients in the balanced chemical equation. The one with the smallest mole-to-coefficient ratio is the limiting reactant. For the reaction of ammonia to nitric oxide, you can calculate the mole-to-coefficient ratio for the ammonia and oxygen like this:

Ammonia has a mole-to-coefficient ratio of 1.47, and oxygen has a ratio of 0.625. Because oxygen has the lowest ratio, oxygen is the limiting reactant, and you need to base your calculations on it.
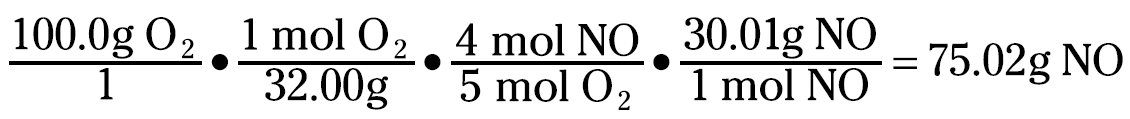
That 75.02 grams NO is your theoretical yield. But you can even calculate the amount of ammonia left over. You can figure the amount of ammonia consumed with this equation:

You started with 100.0 grams of ammonia, and you used 42.58 grams of it. The difference (100 grams - 42.58 grams = 57.42 grams) is the amount of ammonia left over.
 الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


