

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Solutions and Their Concentrations
المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
المصدر:
Fundamentals of Analytical Chemistry
الجزء والصفحة:
9th ed - p67
8-8-2016
1593
Solutions and Their Concentrations
Over the course of history, measurements and their corresponding units were invented at the local level. By necessity of primitive communication and local technology, standards were nearly nonexistent, and conversions among the many systems were difficult. The result was many hundreds of distinct ways of expressing concentrations of solutions. Fortunately for us, the advent of rapid communications technology and the development of efficient travel have forced globalization of measurement science and, along with it, the definition of global measurement standards. No field has enjoyed more benefit in this regard than chemistry in general and analytical chemistry in particular. Even so, we use a number of methods for expressing concentration.
Concentration of Solutions
In the pages that follow, we describe the four fundamental ways of expressing solution concentration: molar concentration, percent concentration, solution-diluent volume ratio, and p-functions.
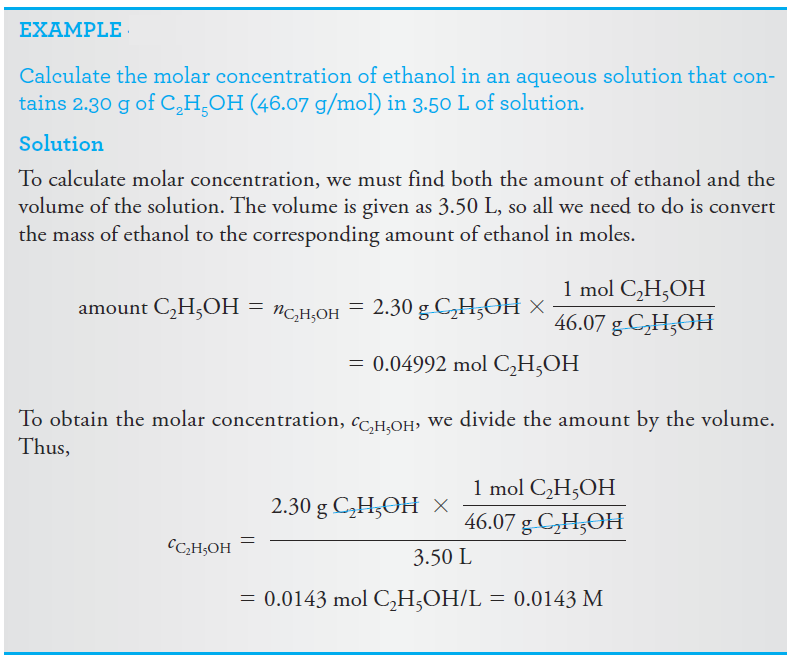
Molar Concentration
The molar concentration cx of a solution of a solute species X is the number of moles of that species that is contained in 1 liter of the solution (not 1 L of the solvent).
In terms of the number of moles of solute, n, and the volume, V, of solution, we write
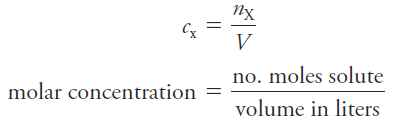
The unit of molar concentration is molar, symbolized by M, which has the dimensions of mol/L, or mol.L-1. Molar concentration is also the number of millimoles of solute per milliliter of solution.
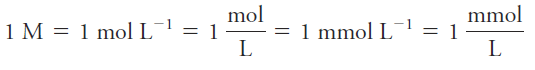
 الاكثر قراءة في التحليل النوعي والكمي
الاكثر قراءة في التحليل النوعي والكمي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















