

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Carbocation Structure and Stability
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th - p208
11-7-2016
5121
Carbocation Structure and Stability
To understand why Markovnikov’s rule works, we need to learn more about the structure and stability of carbocations and about the general nature of reactions and transition states. The first point to explore involves structure. A great deal of experimental evidence has shown that carbocations are planar. The trivalent carbon is sp2-hybridized, and the three substituents are oriented toward the corners of an equilateral triangle, as indicated in Figure 1-1.
Because there are only six valence electrons on carbon and all six are used in the three s bonds, the p orbital extending above and below the plane is unoccupied.
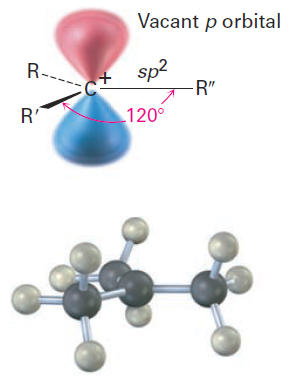
Figure 1-1 The structure of a carbocation. The trivalent carbon is sp2-hybridized and has a vacant p orbital perpendicular to the plane of the carbon and three attached groups.
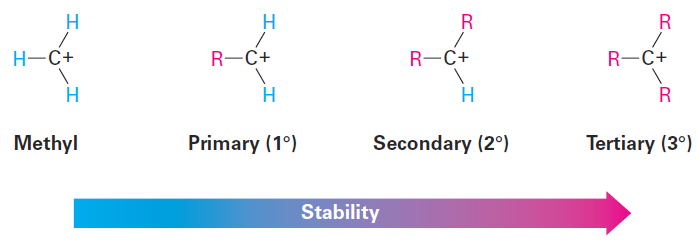
The second point to explore involves carbocation stability. 2-Methylpropene might react with H1 to form a carbocation having three alkyl substituents (a tertiary ion, 3°), or it might react to form a carbocation having one alkyl substituent (a primary ion, 1°). Since the tertiary alkyl chloride, 2-chloro- 2-methylpropane, is the only product observed, formation of the tertiary cation is evidently favored over formation of the primary cation. Thermodynamic measurements show that, indeed, the stability of carbocations increases with increasing substitution so that the stability order is tertiary > secondary > primary > methyl.
One way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by dissociation of the corresponding alkyl halide, R- X → R++ :X-. As shown in Figure 1-2, tertiary alkyl halides dissociate to give carbocations more easily than secondary or primary ones. Thus, trisubstituted carbocations are more stable than disubstituted ones, which are more stable than monosubstituted ones.

Figure 1-2 A plot of dissociation enthalpy versus substitution pattern for the gas-phase dissociation of alkyl chlorides to yield carbocations. More highly substituted alkyl halides dissociate more easily than less highly substituted ones.
The data in Figure 1-2 are taken from measurements made in the gas phase, but a similar stability order is found for carbocations in solution. The dissociation enthalpies are much lower in solution because polar solvents can stabilize the ions, but the order of carbocation stability remains the same. Why are more highly substituted carbocations more stable than less highly substituted ones? There are at least two reasons. Part of the answer has to do with inductive effects, and part has to do with hyperconjugation.
In the present instance, electrons from a relatively larger and more polarizable alkyl group can shift toward a neighboring positive charge more easily than the electron from a hydrogen. Thus, the more alkyl groups attached to the positively charged carbon, the more electron density shifts toward the charge and the more inductive stabilization of the cation occurs (Figure 1-3).
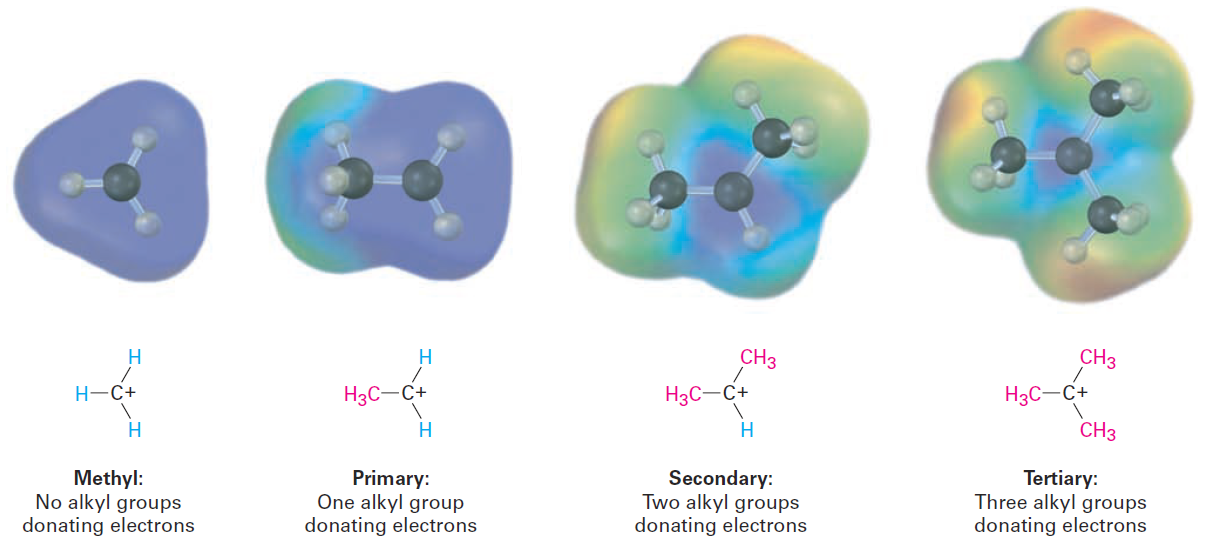
Figure 1-3 A comparison of inductive stabilization for methyl, primary, secondary, and tertiary carbocations. The more alkyl groups that are bonded to the positively charged carbon, the more electron density shifts toward the charge, making the charged carbon less electronpoor (blue in electrostatic potential maps).
The more alkyl groups there are on the carbocation, the more possibilities there are for hyperconjugation and the more stable the carbocation. Figure 1-4 shows the molecular orbital for the ethyl carbocation, CH3CH2+, and indicates the difference between the C-H bond perpendicular to the cation p orbital and the two C-H bonds more parallel to the cation p orbital. Only these roughly parallel C-H bonds are oriented properly to take part in hyperconjugation.
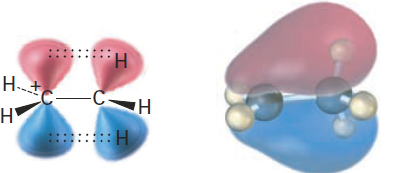
Figure 7-12 Stabilization of the ethyl carbocation, CH3CH2+, through hyperconjugation. Interaction of neighboring C-H σ bonds with the vacant p orbital stabilizes the cation and lowers its energy. The molecular orbital shows that only the two C-H bonds more parallel to the cation p orbital are oriented properly. The C-H bond perpendicular to the cation p orbital cannot take part.
 الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















