Focal Contact
Focal contacts, focal adhesions, or adhesion plaques are cell “junctions” by which many cell types in culture adhere to their substratum, being regions of closest approach between the lower cell surface and the substratum, a separation of approximately 10 nm. Elongated structures that may be 3 to 4 mm in length, they may be recognized as black areas by interference reflection microscopy (Fig. 1), by electron microscopy, or by staining with antibodies against one of their numerous components 1) 2, ) . These contacts are points at which the actin cytoskeleton associates with the cell surface, and are located at the cell-surface termini of actin microfilament bundles, called stress fibers. Focal contacts and stress fibers do not have clear equivalents in vivo, although their molecular composition resembles that of the dense bodies of smooth muscle. Nevertheless, the molecules, molecular interactions, and cellular functions that have been discovered by studying focal contacts are likely to be of great importance in regulating cell behavior in vivo. In tumor cells, disruption of focal contacts is associated with the transformed phenotype (3). The components of focal contacts are involved both in extracellular matrix adhesion and signal transduction.
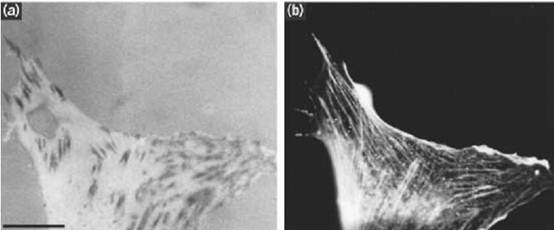
Figure 1. (a, b) Interference reflection image (a) and fluorescent image showing phalloidin staining for F-actin (b) of a cultured fibroblast, illustrating focal contacts and stress fibers. Focal contacts are the elongated black areas in a. Note how the actin stress fibres terminate at focal contacts at their peripheral ends. (Photographs provided by Dr. G. Ireland.) Bar=10 µ m.
The principal adhesive components of focal contacts are integrins (4) (Fig. 2) although they also contain membrane heparan sulfate proteoglycan. These adhesion receptors bind the cell to components of the extracellular matrix, such as fibronectin and vitronectin. Several actin-binding proteins associate with the cytoplasmic side of the plasma membrane at focal contacts. These include a-actinin (2), vinculin (5), talin (6), and tensin (7), the first two of which are also found in intermediate junctions . Talin and a-actinin have been shown to bind to the cytolasmic domains of integrins, particularly the b1 subunit (6, 8). Tensin has actin filament-capping activity. The location of these proteins to focal contacts and their binding interactions are based on a variety of types of evidence, including expression of mutant proteins and in vitro binding between purified, recombinant, or synthetic proteins or peptide fragments. The precise manner in which these proteins interact in focal contacts is not understood. Collectively, they provide a link between the integrin adhesion receptors and the actin cytoskeleton.
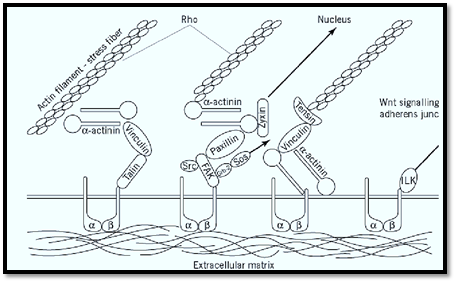
Figure 2. Diagram showing some of the major components of focal contacts and their possible molecular interactions. Sr Grb 2 = growth factor receptor bound protein 2, SOS = son-of-sevenless, ILK = integrin-linked kinase, a = integrin a sub b = integrin b subunit. Arrows indicate signaling pathways, details of which are not shown.
Cells must be able to break and reform focal contacts in order to move. They have been shown to contain the proteinase calpain, which is able to cleave talin and may be involved in modulating junctional adhesion (9). Alternatively, phosphorylation signals generated from within the cytoplasm may modulate the adhesiveness of focal adhesions through regulation of integrin function (10). The oncogenic tyrosine kinase pp60 src and protein kinase C, both present in focal contacts, appears to be able to modulate integrin-mediated adhesion, reducing cell-substratum adhesion (11). Such an adhesive change is likely to be important in tumorgenicity, increasing the invasive properties of cells.
An important function of focal contact components is the transduction of signals from outside the cell. Such signals may be involved in regulating a variety of cellular processes, including motility, cell division, gene expression, and apoptosis. An example of a focal adhesion signaling molecule is the focal adhesion kinase (FAK) (12). This is a tyrosine kinase that is activated by ligand binding to integrins (13). FAK has been shown to bind to the cytoplasmic domain of the b1 integrin subunit and also to the vinculin-binding protein paxillin (14). Phosphorylation of FAK in response to integrin-ligand binding increases its tyrosine kinase activity (15). It is also able to associate with a number of other signaling proteins via its various phosphotyrosine residues. Thus, it binds pp60src via the latter's src homology (SH2) domain (16) and similarly binds growth factor receptor-bound protein, or Grb2 (17). The latter interacts with the guanine nucleotide exchange factor mSOS1, which activates the Ras signaling pathway. FAK may also be involved in regulating focal contact turnover, because fibroblasts from FAK – /– mice show increased numbers of more persistent focal contacts (18) .
Another focal contact-associated signaling molecule is the integrin-linked kinase (ILK), a serine-threonine kinase that can bind directly to the cytoplasmic domains of b1 and b3 integrins (19). Its kinase activity modulates cell–matrix interactions. Overexpression of ILK in epithelial cells results in reduction of tumorgenicity and loss of cell–cell adhesion and anchorage-independent growth. Furthermore, it causes down-regulation of expression of the zonula adherens adhesion molecule E-cadherin, translocation of b-catenin to the nucleus, and formation of a transcriptional regulating complex between b-catenin and the transcription factor LEF-1. Thus, ILK mediates cross-talk between cell–matrix and cell–cell adhesion, as well as regulation of the wnt signaling pathway (20).
Both paxillin and another focal-contact associated protein called zyxin possess so-called LIM domains, zinc-finger motifs that are also found in a number of proteins involved in the regulation of gene expression (21, 22). Paxillin becomes tyrosine-phosphorylated on matrix ligand binding, suggesting that it is involved in signal transduction (21). Zyxin binds to a-actinin, binds to other proteins that have potential signaling functions, such as the vasodilator-stimulated phosphoprotein)VASP ), and has a nuclear export sequence. It has also been shown to shuttle between the focal contact and the nucleus, suggesting that it may provide a direct signaling link between matrix receptors at the cell periphery and gene regulation (23).
Regulation of focal contact formation is also regulated by the small Rho GTPase. Overexpression of Rho in quiescent cells causes focal contact formation and stress fiber assembly, whereas inhibition of Rho leads to focal contact disassembly (24). This aspect of Rho signaling is one of several integrated signaling pathways involving small Gtpases that participate in regulating cell adhesion, cell motility, and actin cytoskeleton.
References
1. J. Heath and G. A. Dunn (1978) J. Cell Sci. 29, 197–212.
2. J. Wehland et al. (1979) J. Cell Sci. 37, 257–273.
3. K. Burridge (1986) Cancer Rev. 8, 18–78.
4. W. T. Chen et al. (1985) J. Cell Biol. 100, 1103–1114.
5. B. Geiger et al. (1980) Proc. Natl. Acad. Sci. USA 77, 4127–4131.
6. A. F. Horwitz et al. (1986) Nature 320, 531–533.
7. A. J. Wilkins et al. (1986) J. Cell Biol. 102–103.
8. C. A. Otey et al. (1990) J. Cell Biol. 111, 721–729.
9. M. C. Beckerle et al. (1987) Cell 51, 569–577.
10. M. Coppolino et al. (1995) J. Biol. Chem. 270, 23132–23138.
11. S. Kellie (1988) Bioessays 8, 25–30.
12. M. D. Schaller et al. (1992) Proc. Natl. Acad. Sci. USA 89, 5192–5196.
13. L. Lipfert et al. (1992) J. Cell Biol. 119, 905–912.
14. J. D. Hildebrand et al. (1993) J. Cell Biol. 123, 993–1005.
15. Burridge et al. (1992) J. Cell Biol. 119, 893–903.
16. B. S. Cobb et al. (1994) Mol. Cell Biol. 14, 147–155.
17. D. D. Schlaepfer et al. (1994) Nature 327, 786–791.
18. D. Ilic et al. (1995) Nature 377, 539–544.
19. G. E. Hannigan et al. (1996) Nature 379, 91–96.
20. A. Novak et al. (1998) Proc. Natl. Acad. Sci. USA 95, 4374–4379.
21. C. E. Turner and J. T. Miller (1994) J. Cell Sci. 107, 1583–1591.
22. I. Sadler et al. (1992) J. Cell Biol. 119, 1573–1588.
23. D. A. Nix and M. C. Beckerle (1997) J. Cell Biol. 138, 1139–1147.
24. A. J. Ridley and A. Hall (1992) Cell 70, 389–399.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


