

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الحيوية

مواضيع عامة في المضادات الحيوية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Polyacrylamide gel electrophoresis
المؤلف:
Clive Dennison
المصدر:
A guide to protein isolation
الجزء والصفحة:
19-4-2016
4112
Polyacrylamide gel electrophoresis (PAGE)
Polyacrylamide, as a medium for gel electrophoresis was introduced by Ornstein. It has the advantage of being a synthetic gel, which is highly reproducible. Moreover, the pore size can be controlled by varying the proportions of acrylamide and the crosslinking agent, bisacrylamide Polyacrylamide can be used as a direct replacement for starch, though it is less conveniently used than starch in the horizontal slab format because polymerization of polyacrylamide is inhibited by oxygen, so it must be cast into a sealed mould.
Disc electrophoresis
Polyacrylamide was first introduced concurrently with a new electrophoretic method called “disc electrophoresis”. This was first conducted in glass tubes, in which the separated protein bands constituted a series of discs. The method also embodied discontinuities in the buffer and gels used and so it may be described as discontinuous electrophoresis, abbreviated to disc. electrophoresis. Today, because of the better cooling and the fact that comparison of different samples is facilitated, disc. electrophoresis is generally conducted in vertical gel slabs. With this layout, the sample is applied to one end of the gel slab and so, unlike with a horizontal slab gel lay-out, only anions or cations, but not both, can be analyzed at one time. The original method of Ornstein5, is an anionic system (i.e. anions are analyzed).
In an anionic system, there is usually a common buffer cation, e.g. Trisí throughout. In the buffer compartments, a buffer with an anionic
component having a pH-dependent electrophoretic mobility is used, e.g. glycine-. Above its pI, the anodic mobility of glycine increases with pH as the proportion of glycine- ions increases.

In the gels, an anionic component having a high, pH-independent mobility, e.g. Cl-, is used.
Two gels are used; a large pore stacking gel and a smaller pore running gel. At the outset, both gels contain Tris-HCl buffer, but at different pH values. The buffer in the stacking gel is of lower pH than that in the gel. A schematic view of the apparatus at the start of an electrophoresis run is shown in Fig. 1. The samples are mixed with sucrose or glycerol to increase their density and are layered directly under the electrode buffer. Upon application of the electrical field, a sharp interface develops between the high mobility so-called “leading ion”, i.e. Cl-, and the less mobile so-called “trailing ion”, i.e. glycine, according to Kohlrausch’s regulating functions. In order to visualize the interface between the leading and trailing ions a small amount of a dye, such as bromophenol blue, may be added to the samples or to the upper electrode solution.

Figure 1. Experimental set-up for discontinuous PAGE.
As the interface moves downward, the protein molecules with mobilities intermediate between Cl- and glycine, will be swept up and concentrated into a very thin band. Within this band, individual proteins will become stacked in order of their mobilities, with those of highest mobility immediately next to the Cl- ions. During the later parts of this so-called stacking phase, therefore, all of the proteins will move downwards at the same speed, i.e. this could be called an isotachophoresis stage (iso = “the same”, “tach” = speed). The concentrated band of protein has a higher density than the surrounding buffer and the system would be convectively unstable were it not stabilized by the stacking gel.
The voltage gradient (-dV/dx) within the separating part of the apparatus will not be uniform but will have a stepwise decrement at the interface between the leading and trailing ions. If proteins are additionally present, the step will be comprised of a number of smaller sub-steps, corresponding to interfaces between the different proteins.
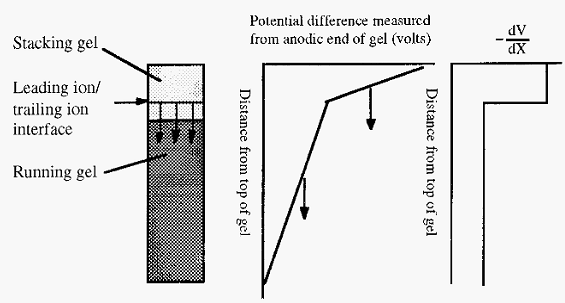
Figure 2. The voltage profile during the stacking phase of disc gel electrophoresis.
Arrows in Fig. 2 indicate the direction of movement of the interface and its associated voltage discontinuity. The voltage gradient is steeper behind the interface than in front. This follows from the fact that all the ions are moving at the same speed. For ions of a low mobility to move as fast as ions of a high mobility, they must be in a steeper voltage gradient. Any protein which falls behind the interface, say by diffusion. will find itself in an area with a steep voltage gradient and will thus be accelerated towards the interface, Conversely any protein which diffuses ahead of the interface will enter an area with a shallow gradient and will slow down and be overtaken by the moving interface. In this way the proteins are focused into thin layers in the interface.
When the interface reaches the junction between the stacking and running gels, two things happen. Firstly the pH increases, resulting in an increase in the mobility of the glycine trailing ion as a larger proportion will exist in the glycine- form at the higher pH. Secondly, the proteins encounter the sieving effect of the small pore running gel. Together these result in the leading ion/trailing ion interface overtaking the stack of proteins, which are left to separate in a linear voltage gradient according to their respective mobilities in the running gel.
The bromophenol blue dye remains with the interface, making its progress easily visible. When the interface reaches near the end of the gel the electric field can be switched off, in the sure knowledge that no proteins will have migrated further than the interface and that all will still be in the gel. The separated proteins can be visualized by staining, for example with Coomassie blue, and destaining.
A number of disc PAGE systems have been described by Jovin.
Isotachophoresis
Although it is not a PAGE system, it is convenient to discus isotachophoresis at this point because it is conceptually related to the stacking phase of disc electrophoresis.
In the later stages of the stacking phase of disc electrophoresis, the proteins are stacked on top of one another in very thin layers (Fig. 3). If the electrophoresis is conducted in a tube, then the thin layers will be in the form of discs.
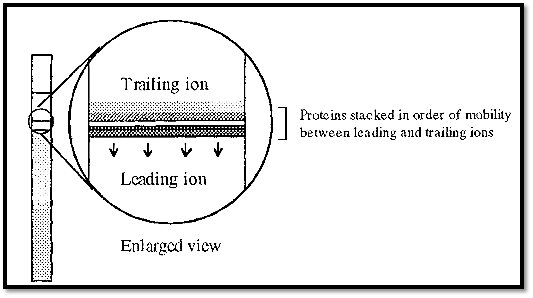
Figure 3. Proteins stacked as thin layers in the stacking phase of disc gel electrophoresis.
The different proteins are in fact separated from one another, although adjacent bands are touching, but this separation is not useful as the bands are so thin that it is impossible to distinguish between them. However, some improvement in the situation could be achieved by making the tube much thinner, so that a given amount of protein would occupy a greater length.
Ultimately, if the separation was carried out in a capillary tube, the bands would occupy a greater length in the capillary tube. In this situation, it is possible to distinguish the different bands. At each interface there is a step change in the voltage gradient, which corresponds to a change in resistance due to the fact that the proteins have different mobilities. This change in resistance at each interface corresponds to a change in heat production and this can be detected with a thermocouple detector. The output is a sharp peak as each interface passes the detector.
In this way the number of proteins present can be determined, but they cannot be identified. If one protein was missing, the others would simply close up and one fewer interface would be detected but it would be difficult to determine which protein was missing. This problem can be overcome by adding a mixture of “ampholytes” to the protein sample. Ampholytes are synthetic polyamino-polycarboxylic acids, in which the amino and carboxyl groups are randomly added to a carbon chain backbone - they are sold under a number of trade names, e.g. Ampholine, Servalyte, Bio-lyte. The result is a mixture of molecules with a range of pI values, resulting in a range of electrophoretic mobilities.
Because of their range of mobilities, there will always be some ampholyte molecules with mobilities intermediate between those of the different proteins and in isotachophoresis these will serve to space the protein molecules a distance apart from one another, and so are known as “spacers”. The separated protein molecules can be detected by UV absorption. Ampholytes will be revisited later in a discussion of isoelectric focusing.
Isotachophoresis is discussed here largely as a conceptual development of the stacking phase of disc electrophoresis. In fact it is a technique which is not much used. However, the concept of conducting electrophoresis in a capillary tube has been highly successful and capillary electrophoresis has become a versatile and popular analytical technique, which can be applied to proteins and other ions. An advantage of the capillary system is that a capillary tube at once controls convection and gives excellent cooling so that high voltages can be used, giving rapid separations. A down-side to capillary electrophoresis is that the apparatus tends to be expensive.
References
Dennison, C. (2002). A guide to protein isolation . School of Molecular mid Cellular Biosciences, University of Natal . Kluwer Academic Publishers new york, Boston, Dordrecht, London, Moscow .
 الاكثر قراءة في عزل البروتين
الاكثر قراءة في عزل البروتين
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















