

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Paper electrophoresis
المؤلف:
Clive Dennison
المصدر:
A guide to protein isolation
الجزء والصفحة:
19-4-2016
3814
Paper electrophoresis
One of the earliest forms of zone electrophoresis for the separation of proteins was paper electrophoresis. In this a strip of filter paper was used as a medium to support a thin layer of buffer. Since the paper served only to support the buffer, paper electrophoresis can be considered as a form of free electrophoresis (as opposed to electrophoresis in a sieving gel) The experimental set-up for paper electrophoresis is shown in Fig. 1.
A strip of filter paper, typically 20 x 150 mm, is marked with pencil to indicate the anodic and cathodic ends and a line is lightly drawn transversely in the middle, where the sample is to be applied. The strip is soaked in buffer, blotted briefly and suspended between supports in the apparatus. Buffer is added to both the anode and the cathode compartments: it is important that the levels in the two compartments are the same to prevent siphoning through the filter paper. The filter paper is connected to the buffer by filter paper wicks, which must be the same width as the filter paper strip, but can be made of several layers of filter paper.

Figure 1. Diagrammatic cross-section of an apparatus for paper electrophoresis.
Sample can be applied as a thin line across the middle of the paper strip, but not within ca. 5 mm of the edges. There are different ways of applying the sample: a simple way, but which requires some manual dexterity, is to use a Pasteur pipette, drawn down to a thin capillary. After application of the sample, the apparatus is sealed with a lid This enables the atmosphere within the apparatus to become saturated with water vapour, thereby preventing evaporation of water from the buffer on the strip. The buffers have a maximal exposed surface area to encourage rapid equilibration of the water vapour. As a safety precaution. the apparatus lid is usually coupled with the electrode connections, so that removal of the lid breaks the electric circuit. Without this precaution, fatal shocks might result from inadvertent contact with the electrode solutions. Electrophoresis is run, usually for a number of hours, typically using a voltage gradient of ca. 10 volts cm-1. To keep electrolysis products away from the protein samples being separated. the buffers in the electrode compartments are separated from the wicks by a baffle system.
After separation, the protein bands are fixed in position and stained with a protein-specific stain, such as Ponceau S or Amido Black, and destained. Paper electrophoresis was used in medical diagnostics and a typical result for the separation of serum is shown in Fig. 2.
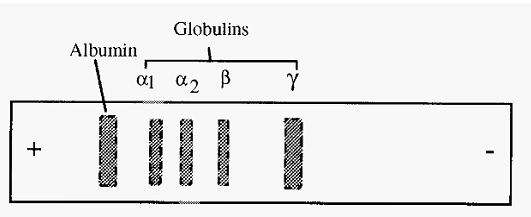
Figure 2. A typical separation of human serum by paper electrophoresis at pH 7.2.
Electroendosmosis
Paper is comprised largely of cellulose, a β →1 linked polymer of glucose. Glucose is hydrophilic due to the polarity of its many -OH groups. Which readily form hydrogen bonds with water. Cellulose as a whole is not water soluble, however, because of its extensive interchain hydrogen bonds. In forming hydrogen bonds with water, the H of the - OH groups of glucose is shared with the oxygen of water, giving the water a δ+ charge and the cellulose oxygen a δ - charge.
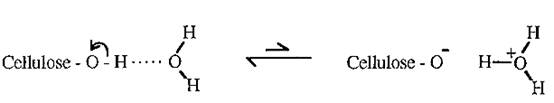
Cellulose thus acquires an overall negative charge, called the “zeta potential”. When placed in an electrical field, a strip of paper (cellulose) tends to move towards the anode, but cannot do so as it is fixed in place.
The hydroxonium ions, H3O+, however, are free to move towards the cathode and do so, resulting in a net drift of the buffer towards the cathode. This drift of the buffer towards the cathode, known as Electroendosmosis, increases the apparent mobility of cations and decreases that of anions. For example, in Fig. 2, γ globulin is seen to have an apparent migration to the cathode. However, the pI of γ globulin is 6.8, and so at pH 7.2 it would be expected to have a net negative charge and a consequent anodic migration. In fact it does have a slight anodic migration but the electroendosmotic flow is faster than this, resulting in the apparent cathodic migration.
References
Dennison, C. (2002). A guide to protein isolation . School of Molecular mid Cellular Biosciences, University of Natal . Kluwer Academic Publishers new york, Boston, Dordrecht, London, Moscow .
 الاكثر قراءة في عزل البروتين
الاكثر قراءة في عزل البروتين
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















