

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Dialysis
المؤلف:
Clive Dennison
المصدر:
A guide to protein isolation
الجزء والصفحة:
17-4-2016
1897
Dialysis
Dialysis is the term used to describe the diffusion of solutes through semi-permeable membranes when the membrane forms the boundary between solutions of different concentrations. The membrane acts as an inert sieve with a certain average pore size. The pores result from the random distribution of the fibres making up the dialysis membrane.
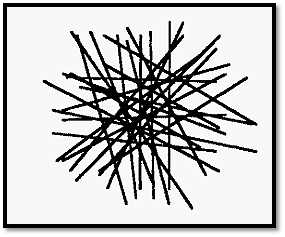
Figure 1. The random distribution of (cellulose) fibres in a dialysis membrane.
A “pore” corresponds to a space bounded by fibres. Clearly the pores are not all of the same size: there is a normal distribution of pore sizes. Molecules with a molecular radius larger than the largest pore size of the membrane will be completely retained while those with smaller radii will pass through more or less easily depending on their size. Fig. 1 shows a 2-dimensional representation but it must be realized that pores are 3- dimensional.
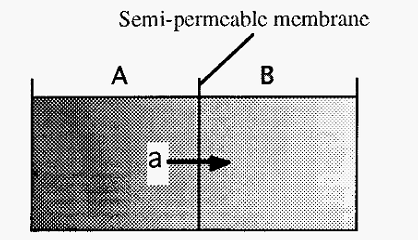
Figure 2. Dialysis across a semi-permeable membrane.
With reference to Fig. 2, consider a small solute “a”, initially in compartment “A” which is separated from compartment “B” by a semi-permeable membrane. As the initial concentration of “a” in A is greater than its concentration in B, a will diffuse from A-----B .
The rate of diffusion will be affected by the following factors:-
• The concentration differential across the membrane. Stirring of both solutions, if possible, and regular changing of solution B will ensure that [a]A >> [a]B and thus the rate of diffusion will be kept at a maximum.
• Surface area. The larger the surface area of the membrane, the faster the overall rate of diffusion. Therefore the membrane area should always be kept at a maximum.
• Solution volume. If the solute molecules have to diffuse a long distance before reaching the membrane, then the rate of dialysis will be relatively slow. Stirring can speed up the rate of transfer to the membrane, but the distance should also be kept to a minimum, i.e. the surface area: volume ratio should be large.
Dialysis is typically used to desalt protein solutions, or to effect a buffer exchange, i.e. to get the protein from one buffer solution into another (Note that “desalting” and “buffer exchange” are really the same process, in the former the second buffer is simply distilled water).

Figure 3. Dialysis using a visking dialysis bag.
Dialysis can be done in various ways, but in the laboratory it is most commonly done using “Visking” tubing. This is a cellulosic material re-constituted into tubular form, dried, and supplied in rolls. A length can be cut from the roll, hydrated by immersion in water for several minutes, and clamped or knotted at one end to form a sealed “dialysis bag”. The protein is introduced into this bag and the open end is sealed by clamping or knotting. The dialysis bag is immersed in a large volume of distilled water or buffer for several hours at 4C to effect exchange of the permeable ions and molecules (Fig. 3), the dialysis solution being changed at intervals (every few hours).
During dialysis, water enters the dialysis bag due to the osmotic pressure of the protein solution. For this reason a dialysis bag must not be filled, but a potential space must be left to accommodate the increasing volume of the protein solution . Note that if the dialysis bag is sealed with knots, the knot should be tightened by pulling only on the outside, not on the bag side of the knot, to avoid stretching the bag and thus distorting the pores.
1. The Donnan membrane effect
The Donnan membrane effect1 describes the phenomenon whereby a charged macromolecule, constrained by a semi-permeable membrane, causes an asymmetrical distribution of permeable ions on either side of the membrane. The net effect is to cause an apparent movement of ions, having the same charge as the protein, away from the protein, i.e. if the protein is positively charged, there will be a lower concentration of small cations in the compartment containing the protein than in the compartment on the other side of the membrane, and vice versa. In buffers, the Donnan effect is not very significant, but when a protein is dialysed against distilled water the Donnan effect can cause significant pH differences on either side of the membrane. This may or may not be significant, depending on the circumstances.
Similarly, ion-exchange resins repel ions of like charge and attract ions of opposite charge. In buffers of low ionic strength, this may cause the pH to be significantly different in the immediate vicinity of the resin substituent groups, compared to that in the bulk of the solution, i.e. cation exchangers, which are negative, will attract cations, including H+ ions, and this will cause a decrease in pH in the immediate vicinity of the resin substituents. With anion exchangers, which are positive, hydroxyl ions are attracted and the pH around the substituents is consequently higher than in the bulk solution.

Figure 4. The Donnan membrane effect.
2 . Counter-current dialysis
A very efficient form of dialysis, often used in automatic analyzers, is counter-current dialysis (CCD). In this, a stream of the solution to be dialyzed is arranged to flow through a thin, convoluted, channel on one side of a dialysis membrane. and the dialysing solution is arranged to flow in the opposite direction through a corresponding thin channel on the other side of the dialysis membrane. In CCD, a maximal concentration difference is thus maintained between the solution being dialyzed and the dialyzing solution. Since thin channels are used, the diffusion distance is small and so there is little need to stir the solutions. A stirring effect can be induced, by flowing the solutions at a high speed so that laminar flow breaks down into turbulent flow, but the period of dialysis per pass is reduced and the benefits, if any, have to be assessed for each case by empirically establishing the optimal flow rate.

Figure 5. Counter current dialysis.
3. Concentration by dialysis (concentrative dialysis)
As mentioned above, a “complication” of dialysis is osmosis, which is the movement of water through a semi-permeable membrane from a solution of low osmotic pressure to a solution of high osmotic pressure. Normally the flow is into the protein solution, so that the protein solution becomes diluted during dialysis against distilled water or a buffer solution: for this reason a dialysis bag is never filled when a protein solution is dialyzed. However, the flow can be reversed and the protein solution concentrated, by dialysing the protein against a solution with a higher osmotic pressure.
The dialysis bag may be simply surrounded by granular sucrose. The water flowing out of the bag will dissolve the sucrose, generating a concentrated sucrose solution with a high osmotic pressure, and this will cause further egress of water from the bag. Alternatively, the dialysis bag may be suspended in a solution of polyethylene glycol (PEG), a hydrophilic polymer.
It will be appreciated that, because water is flowing out of the dialysis bag in such a case, the bag can be filled completely with protein solution before concentrative dialysis. Concentrative dialysis is a specific method in the sense that only macromolecules are concentrated - all buffer salts etc. are not concentrated - but it is non-specific with respect to proteins.
An effect similar to that of concentrative dialysis can be achieved by adding a dry, reversibly hydratable gel (i.e. one that can be dried and reconstituted to have the same structure) such as Sephadex. The Sephadex xerogel will absorb water and, provided it is larger than the exclusion limit of the gel, the protein will be concentrated in the fluid between the swollen gel particles.
4. Perevaporation
A method of concentration using dialysis bags but which is not used much today, is Perevaporation. In this method a dialysis bag containing the protein solution to be concentrated is suspended in a stream of air. Water evaporates from the outside of the bag, keeping the bag and its remaining contents cool. As the water evaporates, all of the non-volatile contents of the dialysis bag are concentrated.
An application of perevaporation which is frequently used today is in the drying of polyacrylamide gels after electrophoresis. Dried gels are mechanically strong and are more easily stored than hydrated gels. To dry the gel, a cellophane membrane is placed on either side of it and the sandwich is suspended in a stream of warm, dry, air until it is completely dry.
References
Dennison, C. (2002). A guide to protein isolation . School of Molecular mid Cellular Biosciences, University of Natal . Kluwer Academic Publishers new york, Boston, Dordrecht, London, Moscow .
 الاكثر قراءة في عزل البروتين
الاكثر قراءة في عزل البروتين
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















