Polylactic Acid (PLA)
PLA is an aliphatic polyester, derived from renewable resources, e. g. corn starch or sugarcane sucrose. It is a polymer produced from lactic acid, which is obtained from the fermentation of various carbohydrate species: glucose, maltose and dextrose from corn or potato starch; sucrose from beet or sugar cane; and lactose from cheese whey.
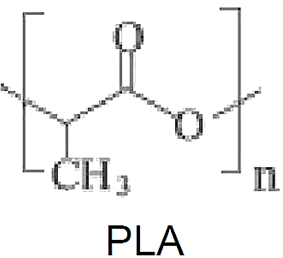
Lactic acid is chiral and has two isomers: the (S)-lactic acid (or L-(+)-lactic acid) and the (R)- lactic acid (ou D-(-)-lactic acid). The lactic acid monomer may be obtained by fermenting carbohydrate crops such as corn, sugar cane, cassava, wheat and barley, being eventually converted to lactide by means of a combined process of oligomerization and cyclization, with the use of catalysts. The synthesis of PLA (polylactate) was explored by Cargill, Hycail, Neste Oy, Shimadzu and Mitsui. Mitsui used a solvent based process to remove water azeotropically in the condensation polymerization process. Neste has obtained high molecular weight PLLA (i. e., L-PLA, or PLA from L-lactic acid) by joining low molecular weight precursors through urethane links. All the others use the dimer lactide process, with lactide ring opening polymerization. In the process using lactides, the additional step of dimerization of lactic acid increases production costs, but improves the control of molecular weight and end groups of the final polymer.
Lactic acid in the L isomeric form may be obtained by fermentation of glucose, while the lactic acid obtained via petrochemistry is in the racemic form (L/D = 1). Through the stereochemical control of lactic acid (ratio of D- and L- optical isomers), one can vary the crystallinity of PLA and also rate of crystallization, transparency, physical properties and even the biodegradation rate. For example, poly(L-lactide), or L-PLA is a semicrystalline polymer with glass transition temperature of 76 °C and melting at 180 °C, while poly(DLlactide), or DL-PLA, is an amorphous polymer with glass transition at 58 °C. DL-PLA is used when it is important to have a homogeneous dispersion of the active species in the single-phase matrix, such as in devices for controlled release of drugs (in the same manner that PLAGA copolymers). L-PLA is preferred for applications where mechanical strength and toughness are required, such as in sutures and orthopedic appliances.
The mechanical properties are somewhat higher than those of polyolefins in general. PLA is a hard material, similar in hardness to acrylics (as methyl methacrylate). Because of its hardness, PLA fractures along the edges, resulting in a product that cannot be used. To overcome these limitations, PLA must be compounded with other materials to adjust the hardness.
The low glass transition temperature is the reason for the limited resistance of PLA to heat, making PLA inadequate for hot drink cups, for example. PLA is suitable for frozen food or for packages stored at ambient temperatures.
PLA have been used in films for packaging, thermoformed and injection moulded disposable rigid containers (for example, food containers and trays), blown flasks and bottles, filaments, and biomedical uses (capsules for drug delivery, fibers for tissues and absorbable/degradable surgical sutures, and internal bone fixation implants). It is a polymer with consolidated use in the medical area, due to its biocompatibility and biodegradability in the human body. PLA-based resins may be modified to adapt to many applications, from disposable food-service items to sheet extrusion, and coating for paper.
Some of the leading manufacturers of PLA are: NatureWorks (Ingeo, capacity of 140 kt per annum), Teijin (BioFront, 1-5 tons per annum, Hisun Biomaterials (5,000 t per annum), Purac/Sulzer/Synbra, SK Chemicals, Biotech, Futerro, Mitsui Toatsu and Shimazu The abiotic degradation of PLA takes place in two stages: a) diffusion of water through the amorphous phase, degrading that phase; and b) hydrolysis of crystalline domains, from the surface to the center. The ester linkages are broken randomly. A semicrystalline material such as poly(L-lactate) presents a hydrolysis rate much lower than that from an amorphous material, such as poly (D,L-lactate), with half-lives of, respectively, one or a few years, and a few weeks. The hydrolysis is self-catalyzed by the acidity of the resulting carboxylic groups.
PLA can not directly be degraded by microorganisms, but requires first abiotic hydrolytic degradation, so that the microorganisms (mainly bacteria and fungi, which form biofilm) can metabolize the lactic acid and its oligomers dissolved in water. Abiotic hydrolysis takes place at temperatures above the glass transition temperature, i. e., at temperatures above 55 oC. Thus PLA is fully biodegradable in composting conditions of municipal waste plants, although it may need a few months to several years to be degraded under conditions of home composting, soil or oceans. Furthermore, the PLA degrading microorganisms are not widespread in the environment.
The polymer passes the tests of compostability, provided that the thickness of the parts do not exceed around 2-3 mm. The extracellular enzymatic degradation consists of two steps: a) the enzyme is adsorbed on the polymer surface, through its binding site; and b) ester bonds are cleaved through the catalytic site of the enzyme. The polymer chain ends are attacked preferentially. The biodegradation rate is a function of the crystallinity and the content of L-monomers. Some enzymes (proteases) that may degrade PLA are proteinase K, pronase and bromelain. Subtilisin, a microbial serine protease, and some mammalian serine proteases, such as α-chymotrypsin, trypsin and elastase, could also degrade PLA.
 الاكثر قراءة في كيمياء البوليمرات
الاكثر قراءة في كيمياء البوليمرات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


