

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
QUANTITATIVE STRUCTURE: ACTIVITY RELATIONSHIPS
المؤلف:
James R Hanson
المصدر:
Chemistry and Medicines
الجزء والصفحة:
p35
29-3-2016
1352
QUANTITATIVE STRUCTURE: ACTIVITY RELATIONSHIPS
Physico-chemical features associated with a structure such as polarity and hydrogen bonding make a considerable contribution to the biological activity. Although there is an overlap, it is possible to discuss these in terms of those factors that affect the transport of a drug to its site of action and those that affect the expression of the biological activity at the site of action. There have been numerous attempts to quantify the relationships between chemical structure and biological activity in terms of measureable physico-chemical parameters and then to use these in a predictive sense in the design of novel drugs.
One of the first attempts to achieve this correlation between biological activity and a measureable physico-chemical parameter was to relate the concentration of various anaesthetics such as diethylether and chloroform required to produce narcosis in test subjects (mice and tadpoles) with the partition co-efficient of the anaesthetic between olive oil and water. This work by Meyer and Overton and by Baum (1899–1901) provided a model for the uptake of the substance by a lipid-like barrier. Many other attempts were made to relate the oil:water partition coefficient to biological activity such as, for example, with a series of barbiturates. The correlation of the oil:water partition co-efficient with biological activity can be a curve with a maximum (Scheme 1). On the one hand too small a partition co-efficient would imply too little material crossing lipid barriers while too high a partition co-efficient would suggest that depots of the drug in fat tissue might be formed. This would lead to insufficient free biologically active material.
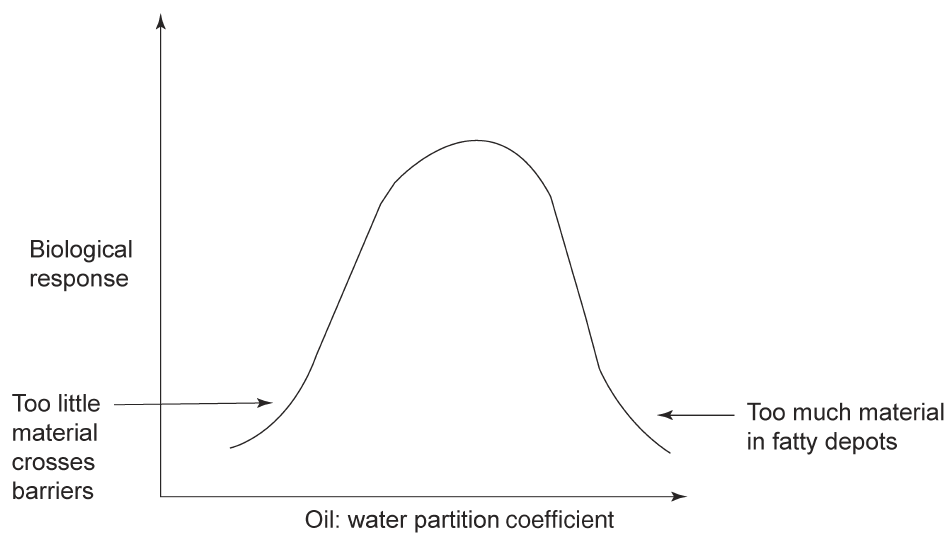
Scheme 1: The origin of an optimum log P
Studies on the local anaesthetic action of the alkamine esters of benzoic acid and p-aminobenzoic acid 2.32 such as amylocaine and procaine 2.31, revealed the relationship of both pK, and hence the concentration of free base, and the partition co-efficient between olive oil and water, to the biological activity.
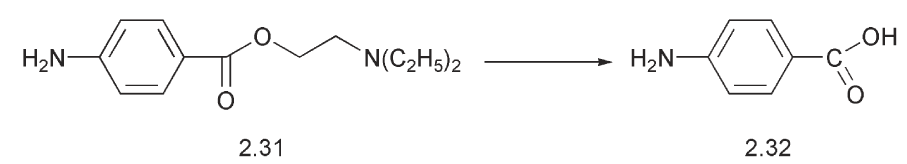
The effect of chain branching, a possible steric factor, was also noted. The olive oil:water partition co-efficient measurements were later replaced by measurements based on octanol:water. By the 1960s, the physico-chemical parameters that might be considered in drug design were grouped into three areas, hydrophobic, electronic and steric.
 الاكثر قراءة في الكيمياء الطبية والدوائية
الاكثر قراءة في الكيمياء الطبية والدوائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















