
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
LASING MEDIUM (NITROGEN LASERS)
المؤلف:
Mark Csele
المصدر:
FUNDAMENTALS OF LIGHT SOURCES AND LASERS
الجزء والصفحة:
p262
24-3-2016
2457
LASING MEDIUM (NITROGEN LASERS)
Diatomic molecules such as nitrogen have vibronic energy levels that combine electronic and vibrational states. The major separation of energy states is caused by a change in the electron energy of the atoms themselves and minor states caused by vibrations of the N2 molecule itself, with v = 0 corresponding to the lowest energy for a given major energy state. The combined effect is that essentially all energy levels consist of a series of closely spaced levels essentially forming a band. As expected, the lasing transition is actually a series of transitions closely spaced to render a primary output centered at 337.1 nm and having a bandwidth of 0.1 nm. These transitions are the result of a change in both electronic and vibrational states of the diatomic molecule.
Excitation of the nitrogen molecule is accomplished by collision of high-energy electrons in a gas discharge. A massive electrical pulse, with currents on the order of thousands of amperes, initiates the process by creating a large flux of electrons with high energies in the laser tube. Electrons strike the nitrogen molecules and excite them to a high vibronic energy state. In the excitation process for this laser, nitrogen molecules do not dissociate into an atomic species but rather, remain as an excited molecular species.
The energy levels of the nitrogen molecule as they apply to this laser are outlined in Figure 1.1. Each level shown is actually a series of vibrational levels dependent on inter nuclear separation. The laser begins when a nitrogen molecule is excited by direct collision with electrons in the discharge to enter the ULL, termed the C3П energy band of the molecule. From the ULL the molecule falls to the LLL (termed the B3П band ), emitting a photon of UV light in the process. Transitions in a normal nitrogen laser operating at 337.1 nm are in the 0–0 band, in which the only levels involved are those with the lowest vibrational state (v = 0). Other transitions are possible in the 0-1 band, where the upper lasing level is the same lowest state (v = 0), but the lower lasing level involved is the v = 1 vibrational state. The transition is a smaller jump than the 337.1-nm transition and hence has lower energy with a wavelength of 357.7 nm, the difference between the v = 1 and v = 0 levels being about 0.2 eV.
After emitting a photon in the lasing transition, the molecule then falls to a metastable state and finally, to the ground state. Although not a four-level laser in the proper sense (the system lacks a pump level since the ULL is pumped by direct electron collision), it is certainly not a three-level system since it does feature a distinct LLL. In this respect the system resembles that of a copper-vapor laser with the inclusion of a metastable state. Each band then consists of multiple closely spaced energy levels, resulting in many possible transitions. In all, molecular nitrogen lases at 61 known wavelengths in the 0-0 band between 336.4903 and 337.9898 nm, with the vast majority of lines clustered around 337.1 nm (with spacing between lines

Figure 1.1. Molecular nitrogen laser energy levels.
typically below 0.005 nm) and a FWHM of the combined output from these lines of about 0.1 nm. Nitrogen lasers can also operate on transitions involving the ionized molecule (N+2) with an output at 427 nm. Such transitions are only possible, though, in a laser operating at high pressures and with large quantities of helium in the gas mixture. This is possible in an excimer laser (also covered in this chapter) by using a helium–nitrogen gas mixture.
The energy levels for molecular nitrogen are far from favorable for lasing, with the upper lasing level having a much shorter lifetime than the lower lasing level. The upper lasing level has a lifetime that is pressure dependent according to
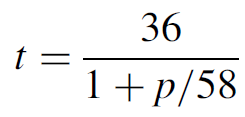
where t is the ULL lifetime in nanoseconds and p is the pressure in torr. So for a nitrogen laser operating at 60 torr, the ULL lifetime is about 18 ns. This is six orders of magnitude shorter than the LLL lifetime of about 10 μs. From the LLL the molecule falls to a metastable state with a longer lifetime yet.
To obtain lasing action from molecular nitrogen there must be a higher population of molecules at the ULL than at the lower level. The level lifetime situation makes CW laser action impossible with this species, but pulsed laser action is still possible provided that the laser mechanism can pump energy preferentially and quickly into the upper lasing level to generate an inversion. In a nitrogen laser this is done by a massive electrical pulse where electron collisions cause the preferential population of the upper energy band first. (Were it not for this effect of being able to pump the upper energy level first, this laser would not work at all.) After about 20 ns, though, this energy will decay to the lower level, population inversion is lost, and lasing action will quickly cease. To make matters worse, nitrogen molecules at the lower state absorb UV light strongly, so gas in the laser channel quickly absorbs laser energy.
On average, then, the pulse length of the nitrogen laser is limited to less than 20 ns (the point where population inversion is no longer possible since half of the molecules in the upper energy state have decayed to the lower state). As we have seen, the lifetime of the upper lasing level depends on the gas pressure in the laser tube. The value of 20 ns quoted assumes a low-pressure laser design where lasing gas is at a pressure between 25 and 60 torr (where 760 torr is 1 atmosphere), but in a TEA laser the pressure is much higher, 760 torr or more, so the lifetime is about 2 ns.
 الاكثر قراءة في بعض تطبيقات الليزر
الاكثر قراءة في بعض تطبيقات الليزر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















