Starch
Starch is a polymer of the group of polysaccharides, to which also belong cellulose, hemicellulose and chitin, among others. Its chemical composition depends on the plant, but generally comprises a mixture of 20-30% by weight amylose and 70-80% by weight of amylopectin.
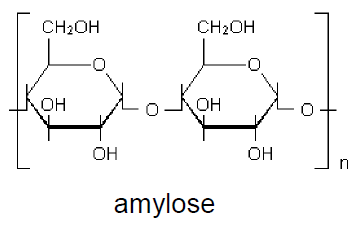
Amylose and amylopectin have molecules formed by glycosidic units bond by α-1,4 ether linkages. Amylose consists of about 200 to 12,000 glycosidic units, forming a straight chain without branching, with secondary (space) helical structure.
Amylopectin consists of about 0.6-2.5 millions of glycosidic units, and is strongly branched. The branches are formed by α -1.6 ether linkages, with average length of 20-30 glycosidic units, occurring every 24-30 units of the straight chain. The chain regions next to the branches are included in the amorphous phase of the material. The chains of the crystalline phase normally have the helix conformation. As a consequence of its molecular structure, starch has lower degree of crystallinity than cellulose, what facilitates its biodegradation.
Just as cellulose, starch is not a thermoplastic material, due to the intensity of the interaction between the molecules by H bonds. Thus heating and shear forces result in degradation before melting. The mechanical properties are poor, both stiffness and toughness, and related properties. Starch is usually obtained from corn, potato and cassava, although it may be obtained from other sources as well. The fact that it is obtained from foods, that require fertile soil for cultivation, has been much questioned, considering that more than 10% of the world population is still undernourished today. From the viewpoint of the life-cycle analysis, products from plant or food wastes are highly favored.
In order to prepare thermoplastic starch, the crystal structure of starch has to be destroyed, either by mechanical working, pressure or heat, or by addition of plasticizers, such as water and glycerin. The gelation of starch is the disruption of the semicrystalline structure of its granules during heating in the presence of water over 90%. The gelation process occurs in two steps. The first, at 60-70 °C, is the swelling of the granules, with little leaching, but with loss of chain organization in crystals. The second above 90 °C, causes the complete disappearance of the granular integrity due to events of swelling and dissolution, making the swollen granules vulnerable to shear.
The destructuring of starch is defined as the melting and the disorganization of the molecular structure of the starch granules and subsequent molecular dispersion in water. The thermoplastic starch product has a starch content above 70% (e. g., 85%), being based on gelled and destructured starch, and on the use of plasticizers, allowing the use of conventional equipment for thermoplastic processing. A problem that can also be an advantage is the high hygroscopicity and solubility in water.
To improve processability, mechanical properties and moisture resistance while maintaining biodegradability, starch may be mixed with aliphatic or aliphatic-aromatic polyesters, such as polycaprolactone (PCL), polylactic acid (PLA) and poly(butylene adipate-coterephthalate) (PBAT), suffering complexing with these polymers.
The complex formed by amylose with the complexing agent is usually crystalline, characterized by an amylose single helix around the complexing polymer. Amylopectin does not interact with the complexing polymer, remaining in the amorphous phase. Starch can also be blended with polyvinyl alcohol (PVOH), for the production of foamed products, such as trays for food. Starch esters reinforced with natural fibers exhibit properties similar to those of polystyrene (PS).
Among the world's leading suppliers are: Novamont (Mater-Bi products) and its licensees (about 80,000 t per year), Rodenburg (Solanyl products, 40,000 t per year), Corn Products, Japan Corn Starch, Chisson, Biotec, Supol, Starch Technology, VTT Chemical, Groen Granulaat and Plantic. The price of the blends of starch with polyesters is about US$ 5 kg-1, while the price of the modified starch is about US$ 1.0 - 1.5 kg-1.
Starch is biodegraded by amylases in a huge variety of different environments. The biodegradation of starch results from the enzymatic attack of the glycosidic linkages, reducing the chain size and producing mono-, di- and oligosaccharides, easily metabolized by biochemical pathways.
Some manufacturers still mix low levels of starch with polyethylene. The rapid biodegradation of the former increases the available surface of the latter to degradation.
Possibly, some of the starch degrading microorganisms may also help with the slow biodegradation of polyethylene. Erlandsson et al have tested a system of starch with LDPE, SBS copolymer, and manganese stearate, after thermo-oxidation at 65 and 95 °C and UV radiation. The starch has stabilized PE regarding thermo-oxidation, but has promoted its photo-oxidation. Among the applications for starch, are films for packaging, shopping bags, garbage bags, mulch films, disposable diapers, foams, foamed trays for food, injection moulded products, blown bottles and flasks, filaments, etc.. The foaming process involves melting (or softening) the polymer and blending it with a foaming agent, typically pentane or carbon dioxide. It is used mainly for polystyrene (PS).
It is the main component of plants, with natural production per year estimated at 7.5 billion tons, and annual human consumption estimated at 200-250 million tonnes. In wood, the cellulose fibrils are joined together by lignin, which is a resin binder.
Cellulose is a polymer made up of about 7,000-15,000 D-glycosidic units (D-glucopyranose residues), joined by β-1.4 ether linkages, that form the cellobiose units (two consecutive glycosidic units), which are repeated along the chain.
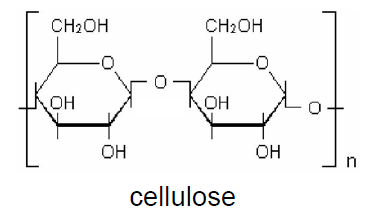
Each glycosidic unit has three hydroxyl groups, that promote strong interactions by hydrogen bonds. The spatial structure allows molecules crystallize in a horizontal plane, forming fibers. As a consequence, cellulose is sparingly soluble and not processable by thermal and mechanical action, i. e. it is not thermoplastic. Cellulose is a rigid material, whose fibers may be used to reinforce other materials. It presents a small elongation capacity. In order to become thermoplastic, it is necessary that about two of the three hydroxyl groups of the glycosidic units be reacted.
Cellulose is biodegraded by the extracellular cellulase enzyme complex, that is induced in most microorganisms. Only a subgroup of cellulase, known as exogluconase, or β-1,4- gluconase, can attack the terminal glycosidic bond, and is effective in degrading the small crystals (crystallites) in which neither water nor enzymes can penetrate. A manufacturer of cellulose based films is Innovia (NatureFlex products).
Cellulose acetate is a thermoplastic derivative obtained by partial esterification of the hydroxyl groups with acetic acid or anhydride. With an average degree of substitution of up to 2.5 of the 3 available glycosidic hydroxyl groups per unit, the polymer is still biodegradable.
The main applications of cellulose are: timber, furniture and fuel; textiles such as cotton; paper, membranes, and explosives. Important cellulose derivatives are cellulose acetate and cellulose acetate butyrate (thermoplastic esters); ethyl, hydroxyethyl and hydroxypropyl cellulose.
 الاكثر قراءة في كيمياء البوليمرات
الاكثر قراءة في كيمياء البوليمرات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


