

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Most probable number ( MPN) method APHA/AWWA/WEF 2005 for total and thermotolerant coliforms and E. coli in water
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
18-3-2016
10608
Most probable number ( MPN) method APHA/AWWA/WEF 2005 for total and thermotolerant coliforms and E. coli in water
Method of the American Public Health Association (APHA), American Water Works Association (AWWA) & Water Environment Federation (WEF), as described in the Standard Methods for the Examination of Water and Wastewater (Hunt and Rice, 2005).
1 - Material required for analysis
Preparation of the sample and serial dilutions
• Magnesium Chloride Phosphate Buffer (Dilution Water)
• Dilution tubes containing 9 ml Magnesium Chloride Phosphate Buffer (Dilution Water)
Total and thermotolerant coliforms count
• Lauryl Sulfate Tryptose (LST) Broth tubes (10 ml)
• Brilliant Green Bile (BGB) Broth 2% with Durham tubes
• E. coli (EC) Broth with Durham tubes
• Pure culture E. coli
• Pure culture E. aerogenes
• Laboratory incubator set to 35 ± 0.5°C
• Water bath set to 44.5 ± 0.2°C
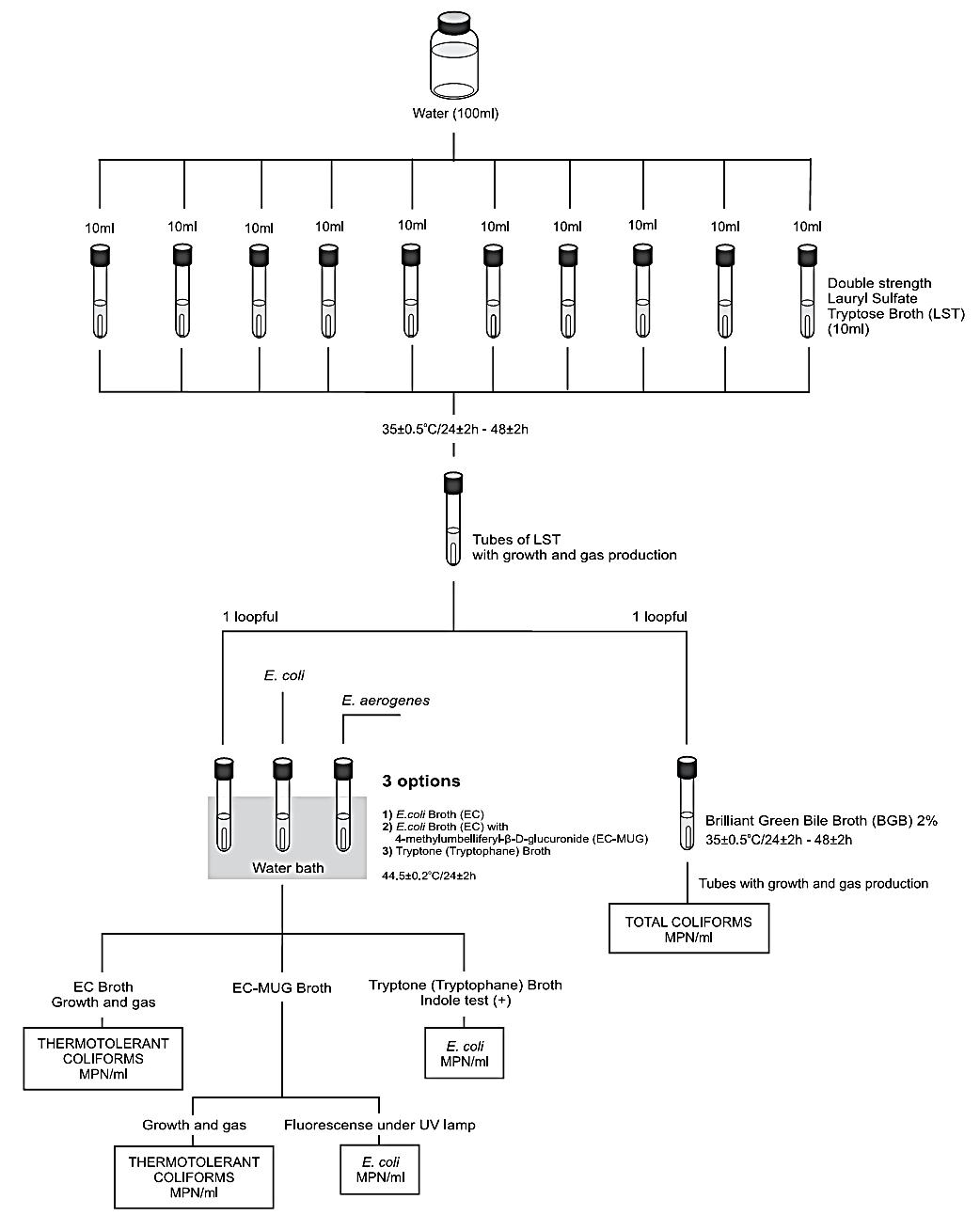
Figure 1 Scheme of analysis for the enumeration total and thermotolerant coliforms and E. coli in water using the Most Probable Number (MPN) method APHA/AWWA/WEF 2005 (Hunt and Rice, 2005).
Thermotolerant coliforms and E. coli count (EC-MUG method for water) (optional)
• E. coli Broth with 4-methylumbelliferyl-β-D-glucuronide (EC-MUG)
• Water bath set to 44.5 ± 0.2°C
E. coli count (indole method for water) (optional)
• Tryptone (Tryptophane) Broth
• Indole Kovacs Reagent
• Water bath set to 44.5 ± 0.2°C
2- Procedure
A general flowchart for the enumeration of total and thermotolerant coliforms and E. coli in water using the Most Probable Number (MPN) method APHA/AWWA/WEF 2005 is shown in Figure 1.
a) Preparation of the samples and inoculation:
Homogenize the sample by shaking vigorously about 25 times. For potable water apply a MPN single dilution test, inoculating 5 × 20 ml aliq-uots of the sample onto 5 × 10 ml of Lauryl Tryptose Broth (LST) triple strength (or 5 × 20 ml of LST double strength LST). It is also possible to inoculate 10 × 10 ml aliquots of the sample onto 10 × 10 ml of Lauryl Tryptose Broth (LST) double strength.
For non-potable water select three appropriate dilutions and apply a MPN multiple dilution test, inoculating five tubes of LST per dilution.
b) Incubation: Incubate LST tubes at 35 ± 0.5°C/24 ± 2 h. Examine tubes and record reactions at 24 ± 2 h for gas (displacement of medium in fermentation vial or effervescence when tubes are gently agitated). Re-incubate gas-negative tubes for an additional 24 h and examine and record reactions again at 48 ± 2 h. Perform confirmed test on all presumptive positive (gas) tubes.
c) Confirmed test for total coliforms: From each gassing LST tube, transfer a loopful of suspension to a tube of Brilliant Green Bile Broth (BGB).
Incubate BGB tubes at 35 ± 0.5°C/24 ± 2 h and examine for gas production, indicative of a positive result. Re-incubate gas-negative tubes for an additional 24 h and examine and record reactions again at 48 ± 2 h. Calculate most probable number (MPN).
Note c.1) Alternative procedure only for polluted water known to produce positive results consistently: If all LST tubes are positive in two or more consecutive dilutions within 24 h, submit to the confirmed test for coliforms only the tubes of the highest dilution (smallest sample aliquots) in which all tubes are positive and the positive tubes of the subsequent dilution. For LST tubes in which the positive result is produced only after 48 h, all should be submitted to the confirmed test for coliforms.
d) Confirmed test for thermotolerant (fecal) coliforms (EC method): From each gassing LST tube, transfer a loopful of the suspension to a tube of E. coli (EC) Broth. Incubate EC tubes at 44.5 ± 0.2°C/24 ± 2 h in a water bath. Examine tubes for growth and gas production, indicative of a positive result. Calculate most probable number (MPN).
Note d.1) Place all the EC tubes in the water bath within 30 min after inoculation. Maintain a sufficient water depth in the water bath incubator to immerse the EC tubes to upper level of the medium. Incubate an EC tube of E. coli and an EC tube of E. aerogenes in the same water bath as positive and negative control.
e) Completed test for thermotolerant coliforms and E. coli (EC-MUG method): From each gassing LST tube, transfer a loopful of the suspension to a tube of E. coli Broth with 4-methylumbelliferyl-β-D-glucuronide (EC-MUG). Incubate EC-MUG tubes at 44.5 ± 0.2°C/24 ± 2 h in a water bath.
Note e.1) Place all the EC-MUG tubes in the water bath within 30 min after inoculation. Maintain a suf-ficient water depth in the water bath incubator to immerse the EC tubes to upper level of the medium. Incubate an EC-MUG tube of a MUG positive strain of E. coli, an EC-MUG tube of Klebsiella pneumoniae (glucuronidase negative) and an EC-MUG tube of E. aerogenes (do not growth at 44.5°C) in the same water bath as controls.
Thermotolerant coliforms: Examine EC-MUG tubes for growth and gas production, indicative of a positive result for thermotolerant coliforms. Calculate most probable number (MPN) following the instructions described in Chapter 4, using a MPN table.
E. coli: Examine al EC-MUG tubes exhibiting growth for fluorescence using a long-wavelength UV lamp (366 nm, preferably six watts). The presence of bright blue fluorescence is considered a positive result for E. coli. Calculate most probable number (MPN) .
f ) Completed test for E. coli (indole method): From each gassing LST tube, transfer a loopful of the suspension to a 5 ml tube of Tryptone (Tryptophane) Broth. Incubate tubes at 44.5 ± 0.2°C/24 ± 2 h in a water bath.
Note f.1) Place all the tubes in the water bath within 30 min after inoculation. Maintain a sufficient water depth in the water bath incubator to immerse the tubes to upper level of the medium. Incubate a tube of an indole positive strain of E. coli, a tube of E. cloacae (indole negative) and an uninoculated tube in the same water bath as controls.
After incubation add 0.2 to 0.3 ml of the Indole Kovacs Reagent to each 5 ml tube of Tryptone (Tryptophane) Broth and examine for appearance of a deep red color in the upper layer (indole test positive). The presence of red color is considered a confirmative result for E. coli. Calculate most probable number (MPN).
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
International Organization for Standardization (2005) ISO 7251:2005. Microbiology of food and animal stuffs – Horizontal method for the detection and enumeration of presumptive Escherichia coli – Most probable number technique. Geneva, ISO.
Hunt, M.E. & Rice, E.W. (2005) Microbiological examination. In: Eaton, A.D., Clesceri, L.S., Rice, E.W. & Greenberg, A.E. (eds). Standard Methods for the Examination of Water & Wastewater. 21st edition. Washington, American Public Health Association (APHA), American Water Works Association (AWWA) & Water Environment Federation (WEF). Part 9000, pp. 9.1–9.169.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















