Presence/absence method MLG/FSIS/USDA 2009 for Listeria monocytogenes in foods
Method of the United States Department of Agriculture (USDA), as described in the Microbiology Laboratory Guidebook Online (USDA/FSIS, 2009). Application: red meat, poultry, egg, and environmental samples.
1 . Material required for analysis
Isolation
• Modified University of Vermont Broth (UVM)
• Fraser Broth or Morpholinepropanesulfonic Acid-Buffered Listeria Enrichment Broth (MOPS-BLEB)
• Modified Oxford Agar (MOX)
• Horse Blood Overlay Agar (HL)
Confirmation
• Brain Heart Infusion (BHI) Broth
• Brain Heart Infusion Agar (BHIA) or Trypticase
Soy Agar with 0.6% Yeast Extract (TSA-YE)
• Trypticase Soy Agar (TSA) with 5% Sheep Blood
• Β-hemolytic Staphylococcus pseudointermedius (ATCC 49444) or Staphylococcus aureus (ATCC 25923) culture
• Rhodococcus equii (ATCC 6939) culture
• β-lysin CAMP factor discs (Remel #21–120, or equivalent) (optional)
• Commercial biochemical identification kit API® Listeria or VITEK 2 Compact or MICRO-ID® Listeria
• Laboratory incubator set to 30 ± 2°C
• Laboratory incubator set to 35 ± 2°C
• Laboratory incubator set to 18–25°C
2 . Procedure
A general flowchart for detection of Listeria monocytogenes in foods using the presence/absence method MLG/FSIS/USDA 2009 is shown in Figure 1.
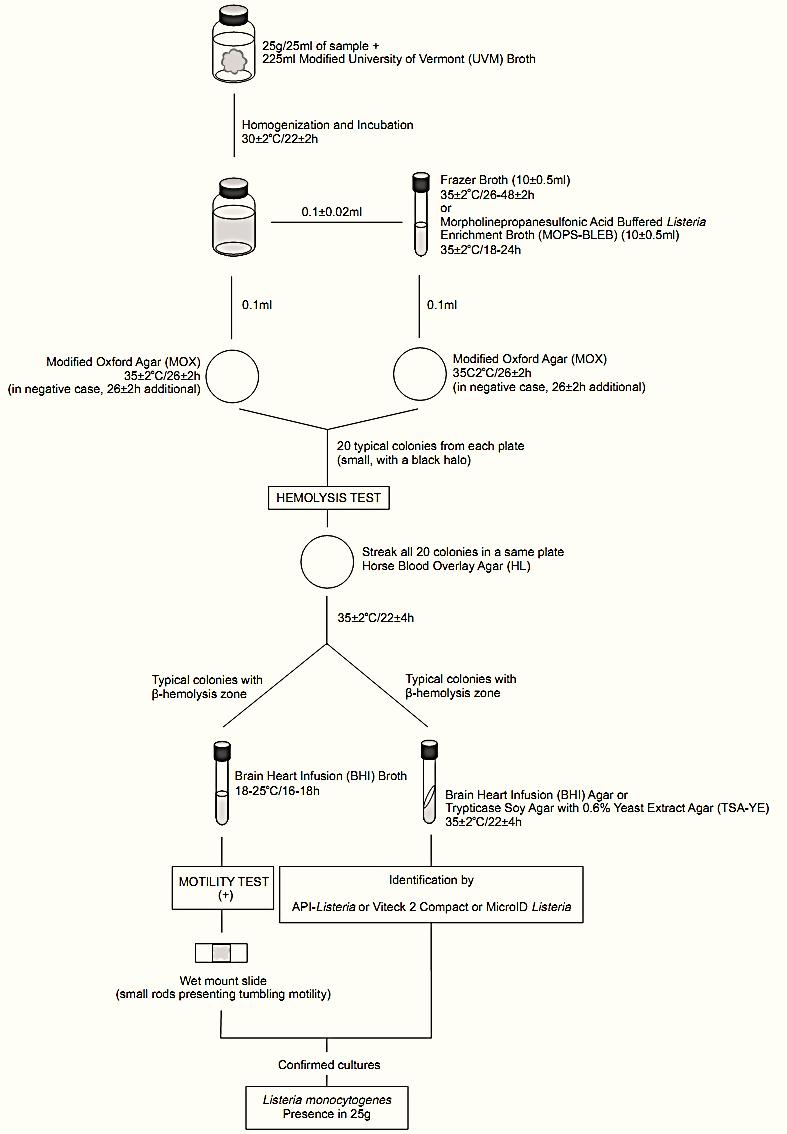
Figure 1 Scheme of analysis for detection of Listeria monocytogenes in foods using the presence/absence method MLG/FSIS/USDA 2009 (USDA/FSIS, 2009).
Safety precautions recommended by MLG: Follow CDC guidelines for Biosafety Level 2 pathogens when using live cultures of L. monocytogenes and work in a class II laminar flow biosafety cabinet. During analysis for L. monocytogenes pregnant women and immunocompromised individuals should not work or entering the laboratory.
a) Primary selective enrichment:
a.1) Presence/absence detection: homogenize 25 ± 1 g of the sample with 225 ± 5 ml of Modified University of Vermont (UVM) Broth. Incubate at 30 ± 2°C/22 ± 2 h.
a.2) Most probable number (MPN) count: homogenize 25 ± 1 g of the sample with 225 ± 5 ml of Modified University of Vermont broth (UVM) (1:10 dilution = 10−1).
Prepare serial decimal dilutions and inoculate three 10 ml aliquots of the 10−1 dilution onto three empty sterile tubes, three 1 ml aliquots of the 10−1 dilution onto three tubes with 9 ml of UVM, and three 1 ml aliquots of the 10−2 dilution onto three tubes with 9 ml of UVM. Incubate the tubes and the remaining 10−1 dilution at 30 ± 2°C/22 ± 2 h. From this point of the procedure continue the analysis separately for each tube and for 10−1 dilution.
Note a.2.1) The aliquots used above (1, 0.1, and 0.01 g) are recommended for foods likely to contain a small L. monocytogenes population (<10/g). For samples with expected count above this level, inoculate higher dilutions.
b) Secondary selective enrichment: After the incubation period inoculate 0.1 ± 0.02 ml of the UVM enrichment into 10 ± 0.5 ml of Fraser Broth or Morpholinepropanesulfonic Acid-Buffered Listeria Enrichment Broth (MOPS-BLEB). Incubate the Fraser Broth at 35 ± 2°C/26 ± 2 h or the MOPS-BLEB at 35 ± 2°C/18–24 h.
If the Fraser Broth show no darkening after 26 ± 2 h of incubation, re-incubate until a total time of 48 ± 2 h. If darkening is evident after 48 h of incubation proceed to selective differential plating (c). If no darkening is evident after 48 h discard the sample as negative for L. monocytogenes.
c) Selective differential plating: From the UVM and from the Fraser Broth or MOPS-BLEB streak a drop (approximating 0.1 ml) onto a plate of Modified Oxford Agar (MOX). Incubate the plates at 35 ± 2°C/26 ± 2 h and verify the presence of typical colonies: small with a dark halo due to esculin hydrolysis. If no suspect colonies are evident, re-incubate the MOX plate for an additional 26 ± 2 h and reexamine.
d) Screening (β-hemolysis and motility test)
d.1) Hemolysis test: Select 20 typical colonies from each MOX plate (or the total number of colonies present, if less than 20) and streak all in a same plate of Horse Blood Overlay Agar (HL). Incubate the plates at 35 ± 2°C/22 ± 4 h and verify the presence of typical colonies of L. monocytogenes, translucent with a small halo of β-hemolysis.
From a well isolated typical colony on HL inoculate a tube of Brain Heart Infusion (BHI) Broth and a tube of Brain Heart Infusion Agar (BHIA) or Trypticase Soy Agar with 0.6% Yeast Extract (TSA-YE). If the suspect colonies on HL are not isolated, purify by streaking on a fresh HL plate and incubate at 35 ± 2°C/22 ± 4 h before inoculating BHI Broth and BHIA or TSA-YE. Incubate BHI Broth at 18–25°C/16–18 h (for motility test below) and BHI Agar or TSA-YE at 35 ± 2°C/22 ± 4 h (for confirmation in the next section e).
After inoculation maintain all the HL plates under refrigeration or at room temperature until the confirmatory step is complete. If the confirmation result is negative for any sample, select additional typical colonies from HL and repeat the confirmation step until at least three isolates from the sample have failed confirmation.
d.2) Motility test: From BHI broth prepare a wetmount and observe microscopically under oil immersion (phase contrast microscopy recommended). Listeria spp. cultures will show an active end-over-end tumbling/rotating motility. If the culture appears mixed purify from the BHI Broth by streaking onto an HL plate and repeat the motility test.
Follow criteria below to select cultures for confirmation:
• Pure culture observed in BHI Broth, but showing cell morphology and motility not characteristic of Listeria spp. – discard as negative for L. monocytogenes.
• Pure culture observed in BHI Broth, showing cell morphology and motility characteristic of Listeria spp. – submit to confirmation.
e) Confirmation: The method MLG/FSIS/USDA 2009 indicates commercially available test system for confirmatory tests, such as MICRO-ID® Listeria, API® Listeria, VITEK® 2 Compact, or equivalent validated.
If the MICRO-ID® Listeria is used, the method MLG/FSIS/USDA require the CAMP test below to reinforce the results.
If the API® Listeria is used and the identification is L. monocytogenes/L.innocua without differentiating the species, the method MLG/FSIS/USDA require genetic identification tests for definitive confirmation (GeneTrak® L. monocytogenes, Gen-Probe AccuProbe® L. monocytogenes or commercial available equivalent).
e.1) Traditional CAMP test (Christie-Atkins-Munch-Peterson Test): This procedure is the same described in the method BAM/FDA 2011, but uses Trypticase Soy Agar (TSA) with 5% Sheep Blood instead of Blood Agar N° 2 with 5% Sheep Blood. The reference cultures indicated are Staphylococcus pseudintermedius ATCC 49444 or Staphylococcus aureus ATCC 25923 and Rhodococcus equi ATCC 6939. If a presumptive culture does not produce typical CAMP reaction after 24 ± 2 h of incubation, reincubate the plate until a total time of 48 ± 2 h. If a culture clearly β-hemolytic on HL does not produce a CAMP positive reaction with Staphylococcus, the method MLG/FSIS/USDA require genetic identification tests for definitive confirmation (GeneTrak® L. monocytogenes, GenProbe AccuProbe® L. monocytogenes or commercial available equivalent).
e.2) β-lysin CAMP test: Place a β-lysin disc in the center of a Trypticase Soy Agar (TSA) plate with 5% Sheep Blood. Use plates with a thinner layer of medium (9 ± 1 ml/plate). Streak up to four cultures to be tested as radiating lines from the disc, without touching the disc with the inoculum. Incubate the plates at 35 ± 2°C/24 ± 2 h.
A positive CAMP test is indicate by hemolytic reaction enhanced near the disc, forming an halo which resembles an arrow. L. monocytogenes, L. seeligeri and L. ivanovii are CAMP positive by this test, but L. ivanovii shows relatively intense β-hemolysis far from the disk. The non-hemolytic species are negative by this test.
If any β-hemolytic culture suspect to be L. monocytogenes does not show a positive result after 24 ± 2 h, reincubate until a total time of 48 ± 2 h. If the result remain negative after 48 ± 2 h, the method MLG/FSIS/USDA require genetic identification tests for definitive confirmation (GeneTrak® L. monocytogenes, GenProbe AccuProbe® L. monocytogenes or commercial available equivalent).
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
USDA/FSIS (2009) Isolation and identification of Listeria monocytogenes from red meat, poultry, egg and environmental samples. In: USDA/FSIS (ed.) Microbiology Laboratory Guidebook, Chapter 8.07. [Online] Washington, Food Safety and Inspction Service, United States Department of Agriculture. Available from: http://www.fsis.usda.gov/Science/Microbiological_Lab_Guide-book/index.asp [Accessed 24th October 2011].
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


