Listeria monocytogenes
1. Introduction
Listeria monocytogenes is an intracellular, foodborne pathogen potentially lethal for humans and animals. The foodborne diseases caused in humans are classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) into two risk groups. For general population is classified in Group I: “diseases of serious hazard, incapacitating but not life threatening, of moderate duration with infrequent sequelae”. For pregnant women and immunocompromised persons is classified in Group IB: “diseases of severe hazard for restricted population; life threatening or resulting in substantial chronic sequelae or presenting effects of long duration”.
2. Taxonomy
In the 9th Edition of Bergey’s Manual of Determinative Bacteriology (Holt et al., 1994) the genus Listeria was placed in group 19, which includes the regular, non-sporing Gram positive rods. In the 2nd Edition of Bergey’s Manual of Systematic Bacteriology the members of group 19 were subdivided into two phyla: the genus Renibacterium was transferred to the phylum Actinobacteria and the other genera of regular Gram positive rods, including Listeria, were transferred to the phylum “Firmicutes” (Garrity and Holt, 2001).
The phylum “Firmicutes” includes the Gram positive bacteria with a low DNA mol% G+C content (<50) (Schleifer, 2009). The genus Listeria is a member of the family Listeriaceae, which also contains the genus Brochothrix (Ludwig et al., 2009).
According to McLauchlin and Rees (2009) Listeria and Brochothrix can be distinguished by a different cell morphology, motility (Brochothrix is non-motile) and colony appearance (Brochothrix colonies do not show blue-green coloration when observed over obliquely transmitted white light). In addition, the optimum temperature for Brochothrix growth is 20–25°C and the range is between 0 and 30°C, while Listeria has an optimum temperature of 30–37°C and grows between <0 and 45°C. L. monocytoges is psychrotrofic and grows well under refrigeration.
The genus Listeria is composed by six species described in the Table 1. Two species are pathogenic; L. monocytogenes is pathogenic to man and to animals and L. ivanovii is pathogenic to animals and rarely occurs in man (Low and Donachie, 1997). These two species contain genes for the virulence factors associated with the bacterial entry into the host cells, the listeriolysin O (LLO) (a 58kDa protein encoded by the gene hly), the phosphatidylinositol-phospholipase C (PI-PLC) (a 33kDa protein encoded by the gene plcA) and the phosphatidylcholine-phospholipase C (PC-PLC) (a 29kDa protein encoded by the gene plcB) (Schmid et al., 2005, Liu, 2006).
All Listeria species are widely distributed in nature (soil, vegetation, sewage, water, animal feed, fresh and frozen poultry, slaughter house wastes, feces of healthy animals including humans) but the disease is predominantly transmitted by the consumption of food contaminated by L. monocytogenes (McLauchlin and Rees, 2009).
The Listeria cells are non-spore-forming Gram positive regular short rods, usually occurring singly or in short chains. All species are motile when cultured at temperatures below 30°C but non-motile when cultured at 37°C. The motility is characterized by tumbling and rotatory movements in wet mounts and by umbrella-like growth in semi-solid motility media (McLauchlin and Rees, 2009).
The Listeria species do not survive heating at 60°C/30 min. The pH range for growth is between 6.0 and 9.0. All species grow in presence of 10% (w/v) NaCl and some strains can tolerate 20% (w/v) NaCl. All species grow in presence of 10% (w/v) and 40% (w/v) bile (McLauchlin and Rees, 2009).
Table.1 Characteristics for differentiating species of the genus Listeria (McLauchlin and Rees, 2009).
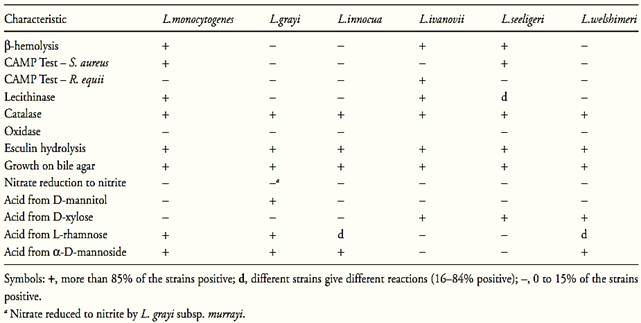
All the Listeria species are aerobic and facultative anaerobic and require carbohydrate for growth. Acid but no gas is produced from glucose and other sugars. Catalase is positive, oxidase is negative, methyl red is positive, Voges-Proskauer is positive, citrate is not utilized and indole is not produced. Urea, gelatin, casein and milk are not hydrolyzed. Sodium hippurate are hydrolyzed (McLauchlin and Rees, 2009).
All Listeria species hydrolize esculin (McLauchlin and Rees, 2009), a naturally occurring glucoside used to detect the enzyme β-glucosidase (esculinase). The product of hydrolysis of esculin, esculetin (6,7-dihydroxycoumarin), can be detected in culture media containing iron salts by the formation of a diffusible brown/black complex. Esculin is the most exploited substrate for β-glucosidase in culture media, but a range of synthetic glucosides is also available, resulting in colored end products.
L. monocytogenes, L. ivanovii and L. seeligeri are hemolytic on blood agar. The remaining three species are non-hemolytic. The zone of hemolysis produced by L. monocytogenes and L. seeligeri are narrow with an indistinct margin. L. ivanovii produces wider zones of hemolysis with sharper edges. Because of the relatively weak hemolytic reactions produced by Listeria, the use of layered blood agar is recommended (McLauchlin and Rees, 2009).
The CAMP (Christie-Atkins-Munch-Peterson) test is used to see the enhancement of hemolysis reaction in presence of Staphylococcus aureus or Rhodococcus equi. L. monocytogenes and L. seeligeri show enhancement of hemolysis (CAMP positive) with S. aureus but not with R. equi. L. ivanovii is CAMP positive with R. equi and negative with S. aureus. L. grayi, L. innocua and L. welshimeri are CAMP negative (McLauchlin and Rees, 2009).
3. Epidemiology
The initial symptoms of the foodborne diseases caused in humans by Listeria monocytogenes simulate influenza, including persistent fever. The infection manifestation can be limited to mild gastrointestinal symptoms such as nausea, vomiting, and diarrhea or may advance to more serious and life-threatening systemic infections These disorders include septicemia, meningitis (or meningoencephalitis), encephalitis, and intrauterine or cervical infections in pregnant women, which may result in spontaneous abortion or stillbirth (FDA, 2009).
The infectious dose is unknown but has been estimated in less than 103 cells. The incubation time for gastrointestinal symptoms is unknown, but is prob-ably greater than 12 hours. The incubation time for the more serious diseases is unknown but may range from a few days to three weeks. The case-fatality rate is high for meningitis (70%), septicemia (50%) and perinatal/neonatal infections (>80%) (FDA, 2009).
The risk groups include pregnant women and their fetus and immunocompromised persons. The elderly and diabetic, cirrhotic, asthmatic, and ulcerative coli-tis patients are also affected, although less frequently (FDA, 2009).
A wide variety of foods may be contaminated with L. monocytogenes including milk and milk products (raw and pasteurized fluid milk, soft-ripened cheese, ice cream), meat and meat products (raw meats, fermented raw-meat sausages, raw and cooked poultry), raw and smoked fish and raw vegetables. The outbreaks and sporadic cases appear to be predominately associated with ready-to-eat foods (FAO/WHO, 2004, FDA, 2009).
4. Methods of analysis
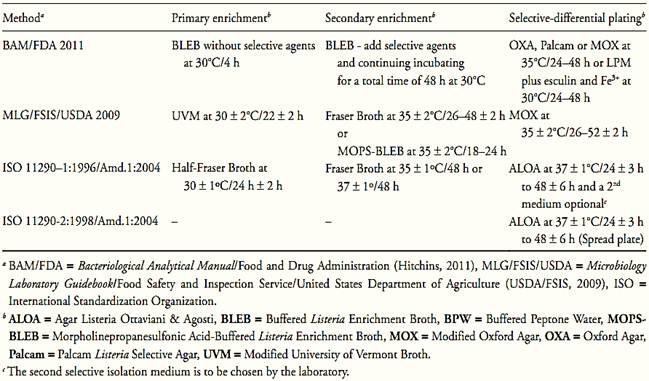
The methods recommended by the different regulatory authorities for L. monocytogenes detection, though they show some variations in the selection of the culture media and the way in which the samples are to be prepared, all basically follow the same steps that can be applied to any type of food. These steps and the media used by Food and Drug Administration (FDA), United States Department of Agriculture (USDA) and International Organization for Standardization (ISO) are summarized in Table 2.
The media used rely on a number of selective agents, including acriflavin, lithium chloride, colistin, cefotetan, moxalactam,and nalidixic acid. The esculin hydrolysis due to β-glucosidase (esculinase) activity is the differential characteristic most used on selective differential plating media to screen suspect colonies. Since all Listeria species are positive for esculin hydrolisis the media based in this characteristic are not specific for L. monocytogenes.
In 2004 ISO 11290-1:1996/Amd.1:2004 and ISO 11290-2:1998/Amd.1:2004 adopted a selective chromogenic plating medium, ALOA ( Agar Listeria Ottaviani & Agosti), to screen suspect colonies. ALOA utilizes two differential substrates, X-Glu ( 5-Bromo-4-chloro-3-indolyl-β-D-glucopyranoside), chromogenic, for the enzyme β-glucosidase, and L-α-phosphatidylinositol, non-chromogenic, for the enzyme phosphatidylinositol-phospholipase (PI-PLC). The cleavage of the chromogenic substrate by β-d-glucosidase results in blue colonies, indicating Listeria spp. The cleavage of the PI-PLC substrate results in a white precipitation halo around the colony, indicating pathogenic Listeria (L. monocytogenes and/or L.ivanovii). ALOA from Oxoid indicates L-α-phosphatidylinositol as substrate for PI-PLC (OCLA Differential Supplement SR0244) or lecithin (Brilliance Listeria Differential Supplement SR0228) for PC-PLC.
BAM/FDA (Hitchins, 2011) also recommends the use of a chromogenic medium, in addition to the chosen esculin-containing selective agar, including ALOA, CHROMagar, BCM® or RAPID'Lmono.
Table.2 Media and incubation conditions recommended by ISO, FDA and USDA methods for Listeria monocytogenes in foods.
According to Reissbrodt (2004) there are two groups of chromogenic plating media for Listeria. The first group includes ALOA and CHROMAgar, which utilizes two differential substrates, one chromogenic for the enzyme β-glucosidase, and one non-chromogenic for the phospholipase(s). CHROMagar™ is a trademark and its formulation is proprietary information. The medium is produced by CHROMagar (France) and by Becton, Dickinson & Company (USA) as BBL™ CHROMagar™.
The second group includes BCM®, Rapid'L.mono and LIMONO-Ident Agar, which utilizes X-phos-Inositol (5-Bromo-4-chloro-3-indoxyl myo-inositol-1-phosphate), a chromogenic substrate for the PI-PLC phospholipase. The formulation of these media is proprietary information and the chromogenic substrate is produced by Biosynth (Switzerland), which provided the following information (Biosynth, 2006). The PI-PLC substrate X-phos Inositol is utilized in BCM® (Biosynth Listeria monocytogenes Plating Media) I and II, in Rapid´L.mono (BioRad) and in LIMONO-Ident-Agar (Heipha, Eppelheim, Germany). Enzymic cleavage of X-phos-Inositol results in turquoise colonies of pathogenic Listeria spp. Non-pathogenic Listeria spp. form white colonies. BCM® Listeria monocytogenes Plating Medium II and LIMONO-Ident-Agar additionally use a selected lecithin–mixture to detect both phospholipases PI-PLC and PC-PLC as a white precipitate halo surrounding the turquoise colonies.
Other methods that already have been officially recognized by the AOAC International are the microbiological test kits described in Table.3.
Table.3 Analytical kits adopted as AOAC Official Methods for Listeria monocytogenes in foods (Horwitz and Latimer, 2010, AOAC International, 2010).

References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
AOAC International (2010). Rapid Methods Adopted as AOAC Official MethodsSM. [Online] Available from: http://www.aoac. org/vmeth/oma_testkits.pdf [Accessed 26th April 2011). Biosynth (2006) BCM® Listeria monocytogenes I and II. [Online] Available from: http://www.biosynth.com/index.asp?topic_id=1 78&g=19&m=256 [Accessed 24th October 2011].
FAO/WHO (2004) Risk assessment of Listeria monocytogenes in ready-to-eat foods: interpretative summary. [Online] Rome, Italy, Food and Agriculture Organization and Word Health Organization. Available from: http://www.who.int/foodsafety/publica-tions/micro/mra_listeria/en/ [Accessed 14th June 2012].
FDA/CFSAN (ed.) (2009) Foodborne Pathogenic Microorganisms and Natural Toxins Handbook “Bad Bug Book”. [Online] College Park, Food and Drug Administration, Center for Food Safety & Applied Nutrition. Available from: http://www.fda.gov/food/foodsafety/foodborneillness/foodborneillnessfoodbornepa-thogensnaturaltoxins/badbugbook/default.htm [accessed 10th October 2011].
Garrity, G.M. & Holt J.G. (2001) The road map to the Manual. In: Boone, D.R. & Castenhols, R.W. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 1. New York, Springer, pp. 119–155.
Hitchins, A.D. (2011) Detection and enumeration of Listeria monocytogenes . In: FDA (ed.) Bacteriological Analytical Manual, Chapter 10. [Online] Silver Spring, Food and Drug Administration. Available from: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/default.htm [accessed 24th October 2011].
Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T. & Williams, S.T. (1994) Bergey’s Manual of Determinative Bacteriology. 9th edition. Baltimore, Williams & Wilkins.
Horwitz, W. & Latimer, G.W. (eds) (2010) Official Methods of Analysis of AOAC International. 18th edition., revision 3. Gaithersburg, Maryland, AOAC International.
ICMSF (International Commission on Microbiological Specifications for Foods) (2002) Microorganisms in Foods 7. Microbiological Testing in Food Safety Management. New York, Kluwer Academic/Plenum Publishers.
International Organization for Standardization (2007) ISO 7218:2007. Microbiology of food and animal stuffs – General require-ments and guidance for microbiological examinations. Geneva, ISO.
International Organization for Standardization (2004) ISO 11290-1:1996/Amd.1:2004. Microbiology of food and animal feeding stuffs – Horizontal method for the detection and enumeration of Listeria monocytogenes – Part 1: detection method. 1st edition:1996, Amendment 1:2004. Geneva, ISO.
International Organization for Standardization (2004) ISO 11290-2:1998/Amd.1:2004. Microbiology of food and animal feeding stuffs– Horizontal method for the detection and enumeration of Listeria monocytogenes – Part 2: Enumeration method. 1st edition:1998, Amendment 1:2004.Geneva, ISO.
Liu, D. (2006) Identification, subtyping and virulence determination of Listeria monocytogenes, an important foodborne pathogen. Journal of Medical Microbiology, 55, 645–659.
Low, J. C. & Donachie, W. (1997) A review of Listeria monocytogenes and listeriosis. Veterinary Journal, 153, 9–29.
Ludwig, W., Schleifer, K.H. & Whitman, W.B. (2009) Family III Listeriaceae fam. nov. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer, p. 244.
McLauchlin, J. & Rees, C.E.D. (2009) Genus I Listeria Pirie 1940. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 244–257.
Perry, J.D., Morris, K.A., James, A.L., Oliver, M & Gould, F.K. (2007) Evaluation of novel chromogenic substrates for the detection of bacterial β-glucosidase. Journal of Applied Microbiology, 102, 410–415.
Reissbrodt, R. (2004) New chromogenic plating media for detection and enumeration of pathogenic Listeria spp. – an overview. International Journal of Food Microbiology, 95(1), 1–9.
Schleifer, K. (2009) Phylum XIII Firmicutes. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer, p. 19.
Schmid, M., Ng, E.Y.W., Lampidis, R., Emmerth, M., Walcher, M., Kreft, J., Goebel, W., Wagner, M. & Schleifer, H. (2005) Evolutionary history of the genus Listeria and its virulence genes. Systematic and Applied Microbiology, 28, 1–18.
USDA/FSIS (2009) Isolation and identification of Listeria monocytogenes from red meat, poultry, egg and environmental samples. In: USDA/FSIS (ed.) Microbiology Laboratory Guidebook, Chapter 8.07. [Online] Washington, Food Safety and Inspction Service, United States Department of Agriculture. Available from: http://www.fsis.usda.gov/Science/Microbiological_Lab_Guide-book/index.asp [Accessed 24th October 2011].
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


