Presence/absence method ISO 21872-1:2007 for presumptive enteropathogenic Vibrio cholerae and Vibrio parahaemolyticus in foods
This method of the International Organization for Standardization is applicable to products intended for human consumption or for the feeding of animals, and to environmental samples in the area of food production and food handling.
1. Material required for analysis
Isolation
• Alkaline Saline Peptone Water (ASPW)
• Thiosulfate Citrate Bile Sucrose (TCBS) Agar
• 2nd V. cholerae and V. parahaemolyticus selective isolation medium plates (chosen by the laboratory)
• Laboratory incubator set to 37 ± 1°C
• Laboratory incubator set to 41,5 ± 1°C
Screening
• Saline Nutrient Agar (SNA)
• Oxidase Kovacs Reagent
• Gram Stain Reagents
• Halotolerance Saline Peptone Water with 10% and 2% of NaCl
• Saline Decaboxylase Broth with 0.5% of Arginine
• Sterile mineral oil
• Laboratory incubator set to 37 ± 1°C
Confirmation by biochemical tests
API 20E or other biochemical identification kit (using 2% NaCl sterile solution to prepare the inoculum) or the material below
• Halotolerance Saline Peptone Water with 0, 2, 6, 8, and 10% of NaCl)
• Saline Decaboxylase Broth with 0.5% of L-Lysine monohydrochloride
• Saline Decaboxylase Broth with 0.5% of L-ornithine monohydrochloride
• Saline Triple Sugar Iron Agar (STSI)
• Saline Tryptophan Broth (5 ml tubes)
• Sterile mineral oil
• Sterile 1% aqueous NaCl solution
• Toluene
• β-Galactosidase reagent (ONPG reagent)
• Indole Kovacs Reagent
• Water bath set to 37 ± 1°C
• Laboratory incubator set to 37 ± 1°C
2 . Procedure
A general flowchart for the determination of Vibrio cholerae and Vibrio parahaemolyticus using the presence/absence method ISO 21872-1:2007/Cor.1:2008 is shown in Figure 1.
Safety precautions recommended by ISO 21872-1:2007/Cor.1:2008: Perform the tests in properly equipped laboratories, under the control of a skilled microbiologist. Take care in the disposal of all contaminated material. ISO 21872-1:2007/Cor.1:2008 recommendations
for the samples storage: According the ISO 21872-1:2007/Cor.1:2008 samples intended to be used for the enumeration of V. cholerae and V. parahaemolyticus should be analyzed immediately or refrigerated (if necessary) for the shortest possible time.
a) Enrichment and selective differential plating
a.1) First enrichment: homogenize m grams of the test sample with 9m milliliters of Alkaline Saline Peptone Water (ASPW). Incubate the flasks in the following conditions: Fresh products at 41.5 ± 1°C/6 ± 1 h; deep-frozen, dried or salted products at 37 ± 1°C/6 ± 1 h.
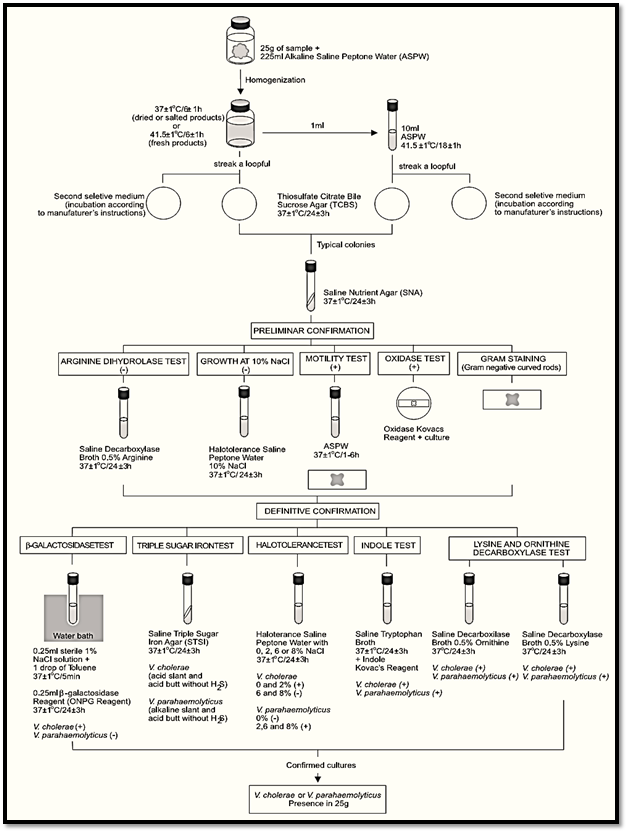
Figure 1 Scheme of analysis for detection of presumptive enteropathogenic Vibrio cholerae and Vibrio parahaemolyticus in foods using the presence/absence method ISO 21872-1:2007/Cor.1:2008.
Note a.1.1) The ISO 21872-1:2007/Cor.1:2008 does not specify the sample quantity to be analyzed and recommends selecting the analytical unit according to the sensitivity required.
Note a.1.2) The described procedure is a presence/absence test that can be adapted for MPN count: homogenize m grams of the test sample with 9m milliliters of Alkaline Saline Peptone Water (ASPW) (1:10 dilution = 10−1). Prepare serial decimal dilutions and inoculate three 10 ml aliquots of the 10−1 dilution onto three empty sterile tubes, three 1 ml aliquots of the 10−1 dilution onto three tubes with 9 ml of ASPW, and three 1 ml aliquots of the 10−2 dilution onto three tubes with 9 ml of ASPW.
Incubate the tubes and the remaining 10−1 dilution at 41.5 ± 1°C/6 ± 1 h (fresh products) or at 37 ± 1°C/6 ± 1 h (deep-frozen, dried or salted products). From this point of the procedure continue the analysis separately for each tube and for 10−1 dilution. The aliquots used above (1, 0.1, and 0.01 g) are recommended for foods likely to contain a small target population (<10/g). For samples with expected count above this level, inoculate higher dilutions.
Note a.1.3) For the examination of several separate samples units of a product lot, whenever there is evidence that the composition will not affect the result for that type of food, the practice of pooling the samples may be utilized (10 × 25 g pooled and diluted in 2.25 liters of ASPW, for example). The ASPW should be pre-warmed to the incubation temperature before inoculation.
a.2) Second enrichment: After 6 ± 1 h of incubation, transfer 1 ml of the first enrichment ASPW culture into a tube containing 10 ml of ASPW and incubate the tubes at 41.5 ± 1°C/18 ± 1 h.
a.3) Selective differential plating: From each ASPW culture (first and second enrichment) streak a loopful onto a Thiosulfate Citrate Bile Sucrose (TCBS) Agar plate. Proceed in the same manner with a second selective isolation medium chosen by the laboratory. Incubate the TSBC plates inverted at 37 ± 1°C/24 ± 3 h. Incubate the second isolation medium plates according to manufacturers’ instructions.
Note a.3.1) Examples for second isolation medium: Sodium Dodecyl Sulfate Polymixin Sucrose (SDS) incubated at 35–37ºC/18–24 h (Vanderzant and Splittstoesser, 1992). Triphenyltetrazolium Chloride Soya Tryptone (TSAT) Agar incubated at 36°C/20–24 h (Kourany, 1983).
b) Screening: Select for screening five typical colonies from each plate. If there are fewer than five colonies, select all. On TCBC the V. cholerae colonies are large (2–3 mm) and yellow (sucrose positive). The V. parahaemolyticus colonies are large (2–3 mm) and green (sucrose negative).
Note b.1) On the SDS second isolation medium cited as example the typical colonies of V. cholerae are yellow and of V. parahaemolyticus are blue-green (Vanderzant and Splittstoesser, 1992). On the TSAT the colonies of V. parahaemolyticus are dark red (reduction of triphenyltetrazolium chloride), smooth, flat, with 2–3 mm in diameter (Kourany, 1983).
Transfer the typical colonies to Saline Nutrient Agar (SNA) slants. Colonies selected from crowded plates should be purified by streaking on SNA Agar plates. Incubate SNA slants or plates at 37 ± 1°C/24 ± 3 h and proceed with the screening tests below (from SNA plates use a single isolated colony for the tests).
b.1) Oxidase test: Using a platinum/iridium loop or glass rod, take a portion of the SNA culture and streak it onto a filter paper moistened with the Oxidase Kovacs Reagent. The appearance of a mauve, violet or deep blue color within 10s indicates a positive reaction. V. cholerae and V. parahaemolyticus are oxidases positive.
b.2) Gram stain and motility test: From each SNA cultures prepare a smear for Gram stain and inoculate a tube of ASPW. Stain the smear. Incubate the ASPW tube at 37 ± 1°C/1–6 h, prepare a wet mount and examine for motility under the microscope. V. cholerae and V. parahaemolyticus are Gram negative, motile.
Note b.2.1) From this point of the procedure the tests may be performed in the sequence described below or the sequence of tests may be performed step-by-step, if desired.
For step by step confirmation use the cultures oxidase-positive, Gram-negative and motile to undertake tests for growth at 10% NaCl (b.3) and arginine dihydrolase (b4). Continue with the other confirmation tests on any colonies that do not show growth in 10% NaCl and give a negative arginine dihydrolase reaction.
b.3) Growth at 10% of NaCl concentration: Pre-pare a suspension from each SNA cultures and inoculate a tube of Halotolerance Saline Peptone Water with 10% of NaCl. Incubate the tubes at 37 ± 1°C/24 ± 3 h. Observation of turbidity indicates growth at the corresponding NaCl con-centration present in the tube. V. cholerae and V. parahaemolyticus do not grow at 10% of NaCl.
Note b.3.1) Inoculate a tube of Halotolerance Saline Pep-tone Water with 2% of NaCl at the same time as a control to check the culture viability.
b.4) Arginine dihydrolase test: From each SNA cultures inoculate a tube of Saline Decaboxylase Broth with 0.5% of Arginine, cover with a layer (1 ml) of sterile mineral oil and incubate at 37 ± 1°C/24 ± 3 h. Turbidity and a violet color after the incubation indicate a positive reaction. Yellow color indicates a negative reaction. V. cholerae and V. parahaemolyticus are arginine dehydrolase negative.
c) Confirmation by biochemical tests: Retain for the biochemical tests the oxidase positive and Gram negative cultures with a positive result in the motility test, a negative result in the arginine dehydrolase test and negative growth at 10% of NaCl.
According the ISO 21872-1:2007/Cor.1:2008 the identification of Vibrio is difficult and it is preferable to obtain confirmation by sending the cultures to a specialist or reference laboratory. The tests described below are presented as a guide to distinguish presumptive V. cholerae or V. parahaemolyticus isolates.
The API 20E (BioMérieux) diagnostic strip or other similar biochemical identification kit also may be used. In this case, follow the manufacturer’s instructions and prepare the inoculum suspending a loopful of the culture into a 2% NaCl solution.
c.1) Halotolerance test: Prepare a suspension from each SNA cultures and inoculate a series of tubes of Halotolerance Saline Peptone Water (0, 2, 6, 8, and 10% NaCl). Incubate the tubes at 37 ± 1°C/24 ± 3 h. Growth (tolerance) at the corresponding NaCl concentration present in the tube is indicated by turbidity.
Note c.1.1) If the 10% and 2% concentrations were tested in the screening step (b.3) it is not necessary to repeat.
c.2) Lysine and ornithine decarboxylase tests: From each SNA cultures inoculate a tube of Saline Decaboxylase Broth with 0.5% of L-Lysine and a tube of Saline Decaboxylase Broth with 0.5% of L-ornithine. Cover with a layer (1 ml) of sterile mineral oil and incubate at 37 ± 1°C/24 ± 3 h. A violet color after incubation indicates a positive reaction. A yellow color indicates a negative reaction.
c.3) Growth on Saline Triple Sugar Iron (STSI) Agar: Inoculate each SNA suspect culture onto STSI tubes by streaking the slant and stabbing the butt. Incubate the tubes with loose cap at 37 ± 1°C/24 ± 3 h. The characteristic reactions for V. cholerae are acid (yellow) slant and an acid (yellow) butt without formation of hydro-gen sulfide (H2S). For V. parahaemolyticus are alkaline (red) slant and acid (yellow) butt with-out formation of hydrogen sulfide (H2S).
c.4) β-Galactosidase test: From each SNA cultures inoculate a tube containing 0.25 ml of sterile 1% aqueous NaCl solution. Add one drop of toluene, shake and incubate at 37 ± 1°C/5 min (water bath). Add 0.25 ml of the β-Galactosidase reagent (ONPG reagent), mix and incubate at
37 ± 1°C/24 ± 3 h (water bath). Examine the
tubes periodically for the development of a yellow color (often after 20 min) indicative of positive reaction. If the yellow color is not observed after 24 h the test is considered negative.
Note c.4.1) As an alternative to the conventional test, commercial paper discs can be used, fol-lowing manufacturers’ instructions: Taxo™
ONPG Discs (BBL 231248/231249), ONPG Discs (Oxoid DD013), ONPG Discs (Fluka 49940).
c.5) Indole test: From each SNA cultures inoculate a 5 ml tube of Saline Tryptophan Broth and incubate at 37 ± 1°C/24 ± 3 h. Test for indole by adding 1 ml of Indole Kovacs Reagent to each 5 ml culture. Appearance of distinct red color in upper layer is a positive test. A yellow brown color is a negative test.
e) Interpretation of results: Consider as presumptive potentially enteropathogenic V. cholerae or V. parahaemolyticus the isolates showing the typical biochemical characteristics presented in the Table 1 above.
Table 1 Guide for the interpretation of presumptive potentially enteropathogenic V. cholerae or V. parahaemolyticus confirmatory tests according to the method ISO 21872-1:2007/Cor.1:2008.

References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Kaysner, C.A & DePaola Jr., A. (2001) Vibrio. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 40, pp. 405–420.
Kaysner, C.A & DePaola Jr., A. (2004) Vibrio. In: FDA (ed.) Bacteriological Analytical Manual, Chapter 5. [Online] Silver Spring, Food and Drug Administration. Available from: http://www. fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm070830.htm [Accessed 3rd November 2011].
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


