

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Presence/absence method APHA 2001 and BAM/FDA 2004 for Vibrio cholerae in foods
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
14-3-2016
5346
Presence/absence method APHA 2001 and BAM/FDA 2004 for Vibrio cholerae in foods
Method of the Food and Drug Administration (FDA), as described in the May/2004 revision of the Bacteriological Analytical Manual Online (Kaysner and DePaola Jr, 2004) and of the American Public Health Association (APHA), as described in the 4th Ed. of the Compendium of Methods for the Micro-biological Examination of Foods (Kaysner and De Paola Jr., 2001).
1. Material required for analysis
Isolation
• Alkaline Peptone Water (APW)
• Thiosulfate Citrate Bile Sucrose (TCBS) Agar
• Modified Cellobiose Polymyxin Colistin (mCPC) Agar or Cellobiose Colistin (CC) Agar (optional)
• Laboratory incubator set to 35 ± 2°C
• Laboratory incubator set to 39–40°C (if available)
• Water bath set to 42 ± 0.2°C (for oysters samples)
Screening
• Arginine Glucose Slants (AGS)
• T1N1 Agar (tubes)
• T1N0 Broth (same formulation of T1N1 without agar and without NaCl) (tubes)
• T1N3 Broth (same formulation of T1N1 without agar and NaCl concentration adjusted to 3%) (tubes)
• 0.5% Sodium Desoxycholate Solution (for string test)
• 0.85% Saline Solution
• Oxidase Kovacs Reagent
• V. cholerae polyvalent 01 and O139 antisera
• Inaba and Ogawa antisera
Confirmation by biochemical tests
• Kit API 20E or equivalent
Biotypes El Tor and Classical differentiation (optional)
• Sheep Blood Agar
• Polymyxin B discs 50 U
2. Procedure
A general flowchart for detection of Vibrio cholerae in foods using the presence/absence methods APHA 2001 and BAM/FDA 2004 is shown in Figure.1.
Safety precautions recommended by method BAM/FDA 2004: Perform the tests in properly equipped laboratories, under the control of a skilled microbiologist. Take care in the disposal of all contaminated material.
BAM/FDA 2004 recommendations for the samples storage: Samples intended for vibrios detection or count should be kept at 7°C to 10°C and analyzed as soon as possible. Direct contact with ice should be avoided. If frozen storage is required, a temperature of −80°C is recommended.
a) Enrichment and selective differential plating:
Homogenize 25 g of sample with 225 ml of Alkaline Peptone Water (APW). Incubate the flasks at 35 ± 2°C/6–8 h.
After 6–8 h of incubation, inoculate a loopful from the surface pellicle of APW onto a Thiosulfate Citrate Bile Sucrose (TCBS) Agar plate and incubate the TSBC plate at 35 ± 2°C/18–24 h. A plate of Modified Cellobiose Polymyxin Colistin (mCPC) Agar or Cellobiose Colistin (CC) Agar may also be included. If used, incubate the mCPC or CC plates at 39–40°C/18–24 h (preferably) or 35–37°C/18–24 h if an incubator set to 39–40°C is not available.
For samples processed by heating, freezing or drying re-incubate the APW overnight and repeat the plating on TCBS (and mCPC or CC, if used).
For raw oysters, include a second flask with 25 g of product plus 2475 ml of APW. Incubate the APW at 42 ± 0.2°C/18–21 h in a water bath and proceed with the plating on TCBS (and mCPC or CC, if used) from this flask too.
Note a.1) If the enumeration is desired, use the most prob-able number (MPN) technique: Homogenize 25 g of sample with 225 ml of Phosphate Buffered Saline (PBS). From this first dilution (10−1) prepare decimal serial dilutions. Select three appropriate dilutions and inoculate three 1 ml portions of each dilution onto three tubes with 10 ml of APW. From this point of the procedure continue the analysis separately for each APW tube. For oysters include a second series of APW tubes for the incubation at 42°C.
b) Screening: Select for screening three or more typical colonies from each plate. Typical colonies of V. cholerae on TCBS are large (2 to 3 mm) and yellow (sucrose positive). On mCPC or CC the typical colonies are green to purple.
Transfer the typical colonies to T1N1 agar slants. If selected from crowded plates purify the culture by streaking on T1N1 Agar plates. Incubate T1N1 slants or plates at 35 ± 2°C/overnight and proceed with the screening tests below (from T1N1 plates use a single isolated colony for the tests).
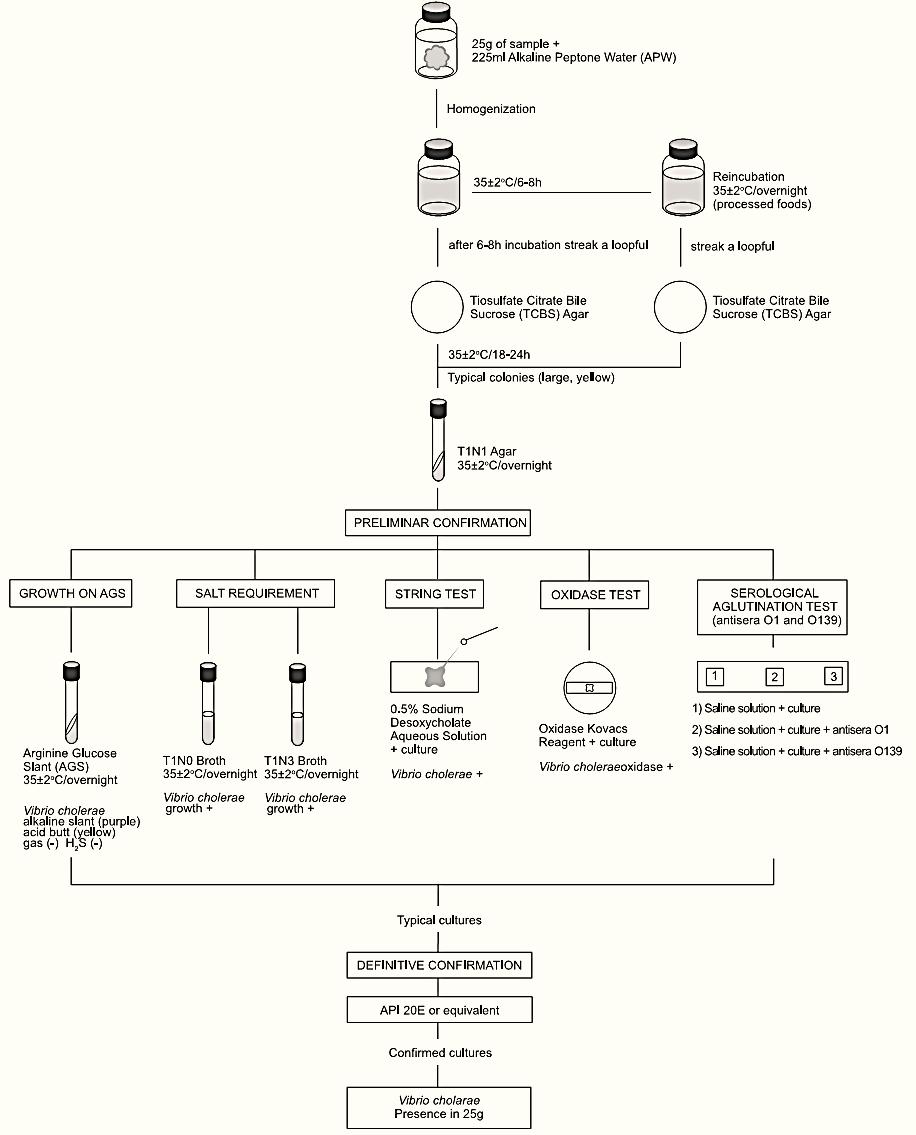
Figure.1 Scheme of analysis for detection of Vibrio cholerae in foods using the presence/absence methods APHA 2001 and BAM/FDA 2004 (Kaysner and DePaola Jr, 2001, Kaysner and DePaola Jr, 2004).
b.1) Growth on Arginine Glucose Slant (AGS): From the T1N1 cultures inoculate tubes of AGS by streaking the slant and stabbing the butt. Incubate the AGS tubes at 35 ± 2°C/overnight (with the caps slightly loosened).
The typical characteristics of V. cholerae (and V. mimicus) on AGS are purple (alkaline) slant and yellow (acid) butt (arginine not hydrolyzed) without gas or H2S.
b.2) Salt requirement test: From the T1N1 cultures inoculate tubes of T1N0 and T1N3 broths. Incubate the tubes at 35 ± 2°C/over-night. V. cholerae does not require NaCl and grows in T1N0 (without NaCl).
b.3) String test: From the T1N1 cultures emulsify a loopful in a drop of 0.5% Sodium Desoxycholate Solution. Examine within 60s for cells lyse (loss of turbidity) which allows the DNA to string when a loopful is lifted (up to 2–3 cm) from the slide. V. cholerae is positive.
b.4) Oxidase test: Using a platinum/iridium loop or glass rod take a portion of the T1N1 growth and streak it onto a filter paper moistened with the Oxidase Kovacs Reagent. The appearance of dark purple color within 10s indicates a positive reaction. V. cholerae (and V. mimicus) are oxidase positive.
b.5) Serologic agglutination tests: In three sections (about 1 × 2 cm) marked with a wax pencil on the inside of a glass Petri dish or on a glass slide add one drop of 0.85% Saline Solution. From the T1N1 cultures emulsify a loopful in each drop and observe for auto-agglutination. If auto-agglutination does not occur, add one drop of polyvalent V. cholerae O1 antiserum to one section and one drop of anti-O139 antiserum to another section. Tilt the mixture in back-and-forth motion for one minute and observe for agglutination against a dark background. If the test is positive, repeat separately with Ogawa and Inaba antisera. The Hikojima serotype reacts with both antisera.
c) Confirmation by biochemical tests: Use the API 20E (BioMérieux) diagnostic strip or an equivalent biochemical kit for the identification and confirmation of the isolates. Follow the manufacturer’s instructions.
d) Differentiation of El Tor and Classical biotypes (optional)
d.1) Beta- hemolysis: From the T1N1 cultures inoculate Sheep Blood Agar plates and incubate the plates at 35 ± 2°C/18–24 h. Exam-ine for beta-hemolysis, indicated by a clear zone around the growth of the culture. El Tor strains are β-hemolytic and Classical strains are not hemolytic.
d.2) Polymyxin-B sensitivity: From the T1N1 cultures inoculate T1N1 agar plates and place a 50 unit disc of polymyxin-B on the surface of each plate. incubate the plates inverted at 35 ± 2°C/overnight. Classical strains are sensitive to polymyxin-B, showing an inhibition zone >12 mm. El Tor strains are resistant.
e) Enterotoxigenity: For these tests it is preferable to send the cultures to a specialist or reference laboratory.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Kaysner, C.A & DePaola Jr., A. (2001) Vibrio. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 40, pp. 405–420.
Kaysner, C.A & DePaola Jr., A. (2004) Vibrio. In: FDA (ed.) Bacteriological Analytical Manual, Chapter 5. [Online] Silver Spring, Food and Drug Administration. Available from: http://www. fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm070830.htm [Accessed 3rd November 2011].
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















