Homogenization of samples and withdrawal of the analytical unit
The analytical unit is the amount of material with-drawn from a sample to be subjected to one or more tests. The number of analytical units that should be withdrawn and the amount of material of each analytical unit depends on the number and types of tests that will be performed on the same sample. In general, the following items are necessary:
a) Analytical units for presence/absence tests with enrichment in specific broth. One analytical unit is required for each test (Salmonella, Listeria and others). The quantity of material of each of these analytical units is defined in the chapters specifically dedicated to these tests.
b) Analytical units for tests requiring differentiated treatment of the sample. One analytical unit is required for each test (commercial sterility, bacterial spore counts, thermo resistant mold counts and others). The quantity of material of each of these analytical units is also defined in the chapters specifically dedicated to these tests.
c) Analytical units for general quantification tests. General quantification tests usually comprise total aerobic mesophilic or psychrotrophic counts and count of yeasts and molds, lactic acid bacteria, enterococci, Enterobacteriaceae, coliform and/or Escherichia coli, Staphylococcus aureus, Bacillus cereus, Clostridium perfringens and Pseudomonas sp. These tests are performed with the same analytical unit, which, most commonly, consists of 25 grams or milliliters of the sample. ISO 6887-1:1999 recommends that the analytical unit be, at least, 10 g for solid samples or 10 ml for liquid samples. recommends that the minimum amount or volume of the analytical unit be at least 50 g for solid foods and 10, 11 or 50 ml for liquid products. However, in the specific chapters, the recommended amount for most cases is 25 g or less. Thus, analytical units of 25 g meet the requirements of ISO 6887-1:1999 and, also those of the Compendium, for most tests.
There are differentiated cases in which the analytical unit must be greater or smaller than specified here.
Before withdrawing the analytical unit(s), the content of the sample should be well homogenized, to ensure that the portion to be removed will be representative for the material as a whole. The procedures to achieve good homogenization are different for liquid products, solid products and products with a predominantly sur-face contamination, as will be further specified in the following sections.
Procedure for homogenization and withdrawal of analytical units from liquid products
If the liquid product (viscosity not greater than that of milk) is filled in containers with enough inner space to allow for agitation, invert the packaging 25 times. If the container is filled to more than two-thirds of its inner space, invert the package 25 times in a 30 cm arc within seven seconds. If there is not enough free space for agitation, then use a second, sterile container and transfer the sample from one container to the other, for three consecutive times. If foam is formed, let it sub-side by standing until totally dispersed. As for gasified samples (carbonated soft drinks and similar products), transfer the content to a sterile container with a wide mouth and, with the cap slightly open, agitate using a shaker until the gas is completely expelled (this step is unnecessary if the analytical unit is transferred directly to the filtration flask, in the tests using the membrane filtration method).
Withdraw the analytical unit with a pipette, inserting the tip of the pipette to a depth not greater than 2.5 cm below the surface of the liquid. The measurement should be volumetric and the time interval between the homogenization of the sample and the withdrawal of the analytical unit should not exceed 3 min. The Compendium does not set a limit for the uncertainty of the measurement of the volume, which, according to ISO 6887-1:1999 should not be greater than 5%.
Procedure for homogenization and withdrawal of analytical units from solid or concentrated liquid products
In the case of solid or concentrated liquid products, which defines the procedures most appropriate for homogenizing and withdrawing the analytical unit of different types of foods. The Compendium (Midura & Bryant 2001) recommends that the uncertainty of mass or weight measurement be not greater than 0.1 g. ISO 6887-1:1999 recommends this measurement uncertainty not to exceed 5%. The interval between homogenization and the withdrawal of the analytical unit should not surpass 15 minutes.
If the sample is frozen, the Compendium (Midura & Bryant, 2001) recommends thawing in the original packaging under refrigeration temperatures (≤4.4°C( for no longer than 18 h. Alternatively, higher temperatures may be used, but not higher than 40°C and for no longer than 15 min. In this case, frequent agitation of the sample is required to facilitate thawing. The use of a controlled temperature water bath and agitation is recommended. ISO 6887-4:2003/Cor.1:2004 recommends thawing under refrigeration (0 to 4°C) for no longer than 24 hours, in the original packaging. Alter-natively, higher temperatures may be used (18 to 27°C), but for no longer than 3 h. In the case of large blocks of frozen foods, which cannot be thawed under the conditions described above, the procedure recommended by ISO 6887-2:2003 for large pieces of meat may be followed. Utilize an electric drill (fitted with a previously sterilized drill bit) in combination with a sterile funnel. Insert the drill bit in the funnel (the lower opening inside diameter of which should be only slightly greater than the diameter of the drill bit) and position the bit onto the point of the block from which a sample should be taken. Turn on the drill and scrapings of the frozen food will move towards the surface and will accumulate in the funnel, from where the required number of analytical units can be taken.
If the sample is heterogeneous, consisting of different layers, each of which of a distinct and clearly different composition (filled cakes, pies, desserts, and other ready-to-eat food), the analytical unit should be put together using portions of the different layers, taking into account the actual proportion of each layer in the product. Alternatively, homogenize the entire content of the sample and withdraw the analytical unit from the macerate. If a blender is used for homogenizing, ISO 6887-4:2003/Cor.1:2004 recommends homogenization time not to exceed one minute, in order to avoid excessive heating. A third option is to withdraw separate and distinct analytical units from each layer and analyze them, separately. If the amount of sample sent for analysis is smaller than the analytical unit(s) required, the Compendium (Midura & Bryant, 2001) recommends subjecting half of the available amount of sample material for analysis and reserve the other half as a counter-sample. If homogenization is done using a blender, the quantity of sample plus diluent (first dilution 10–1) in the jar of the blender should be sufficient to cover the cutting blades of the apparatus. For meat products, ISO 6887-3:2003 recommends using all of the material for the tests.
Procedure for withdrawing
The analytical unit using the surface swabbing technique The surface swabbing technique applies to foods of which most microbial contamination is predominantly present or concentrated on the surface, such as bovine, swine, poultry and fish carcasses. It also applies to the analysis of the surfaces of pieces of equipment, tables, utensils and packaging.
Rubbing can be done with sterile swabs or, if the area to be sampled is large, with sterile sponges. This material can be purchased in individual, sterile pack-ages. The sponges may be replaced by sterile cotton pads, prepared in the laboratory. The swabs may also be prepared in the laboratory, with wooden shafts of approximately 15 cm long by 3 mm in diameter and the absorbent part in cotton measuring approximately 2 cm in length by 5 mm in diameter.
A -Swab sampling
Prepare tubes or flasks with 10 ml of an appropriate diluent. The Compendium (Midura & Bryant, 2001( recommends 0.1% Peptone Water (PW) or Butter-field’s Phosphate Buffer and ISO 6887-1:1999 rec-ommends Saline Peptone Water (SPW) or Buffered Peptone Water (BPW). Remove the swab from its sterile package, holding it by the shaft at the edge opposite to the cotton tip. Moisten the cotton in the diluent, pressing it against the walls of the flask to remove any excess liquid.
Using a sterile frame of 50 cm2 in size, delimit the area to be sampled, holding the frame firmly against the surface. Rub the swab with pressure, moving from left to right and then from bottom to top. Rotate the cotton swab tip continuously as you wipe, so that the entire surface of the cotton comes into contact with the sample. Upon completion of the rubbing or wiping, transfer the swab to the tube or flask containing the diluent, breaking off the hand-manipulated part of the wooden shaft against the inside of the flask tube, before immersing the remainder of the swab in the diluent. Repeat this procedure one more time, covering the same sample surface area, using a dry swab this time. Place and keep the second swab in the same flask or tube containing diluent. The liquid collected by the swabs can be used both in general quantification tests as in presence/absence tests, which require enrichment in differentiated broth (in the second case, follow the guidelines and instructions in each of the specific chapters). This procedure samples a total surface area of 50 cm2 and each milliliter of diluent, upon removal of the swabs, corresponds to 5 cm2 of the sampled surface. Both the sampled surface area as the volume of diluent may vary, in accordance with the needs or the characteristics of the sample.
For the swabbing of half bovine or swine carcasses using the same procedure, ISO 17604:2003/Amd.1:2009 recommends sampling the points indicated in Figures 1 and 2. Use one swab for each point and, between one point and the next, immerse the frame in ethanol 70% and flame-sterilize. The swabs may be placed and kept in one and the same flask containing a total volume of diluent corresponding to a multiple of 10 ml diluent for each pair of swabs.
B- Sponge sampling
Prepare tubes or flasks with 25 ml of one of the diluents recommended for swabs. Open the plastic bag containing the sterile sponge (or cotton pad) and add an amount of diluent sufficient to moisten the sponge, without leaving behind any visible excess fluid. Hold the bag by its outside surface and massage the sponge to moisten it evenly. Thoroughly wash your hands before putting on a pair of sterile gloves and remove the sponge from the bag.
Using a sterile frame measuring 10 × 10 cm, delimit the area to be sampled by holding the frame firmly against the surface. Rub the sponge under pressure, moving it 10 times from left to right and 10 times from bottom to top. Upon completing this procedure, place the sponge back again into the bag and add the remain-der of the diluent, until completing 25 ml.
The liquid collected by the sponges can be used both in general quantification tests as in presence/absence tests, which require enrichment in differentiated broth (in the second case, follow the guidelines and instructions in each of the specific chapters). This procedure samples a total surface area of 100 cm2 and each milliliter of diluent, after the sponge is removed, corresponds to 4 cm2 of the sample surface. Both the sampled surface area as the volume of diluent may vary, in accordance with the needs or the characteristics of the sample.
Procedure for withdrawing
the analytical unit using the surface washing technique The surface washing technique is used for taking food samples of which most microbial contamination is pre-dominantly present or concentrated on the surface, such as whole poultry carcasses, poultry cuts, fish, egg shells, grains, seeds, nuts and peanuts, which may be immersed in an adequate diluent contained in a sterile bag. The method is also used for the analysis of pack-ages that can be closed and agitated with the diluent inside, for washing the package and collecting the sample to be examined.
A- Procedure for washing poultry carcasses
The following procedure is from MLG/FSIS (2011) to be used for the simultaneous examination of Salmonella and other microorganisms. It is also recommended by ISO 17604:2003/Amd.1:2009. Aseptically drain excess fluid from the carcass and transfer the carcass to a sterile plastic bag. Pour 400 ml of Buffered Peptone Water (BPW) into the cavity of the carcass contained in the bag. Rinse the bird inside and out with a rocking motion for one minute (ca. 35 RPM). This is done by grasping the broiler carcass in the bag with one hand and the closed top of the bag with the other. Rock the carcass with a reciprocal motion in about an 18–24 inch arc, assuring that all surfaces (interior and exterior of the carcass) are rinsed. Transfer the sample rinse fluid to a sterile container. Use 30 ± 0.6 ml of the sample rinse fluid obtained above for Salmonella analysis. Add 30 ± 0.6 ml of sterile BPW, and mix well.
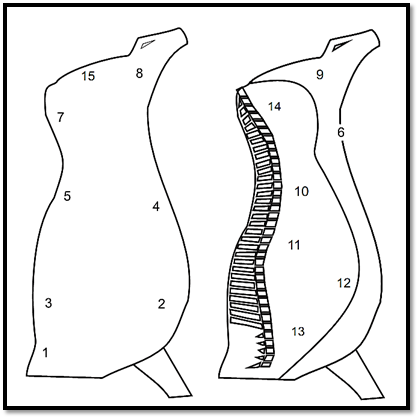
Figure 1 . Points recommended by ISO 17604:2003/Amd.1:2009 for swab sampling of bovine carcasses.
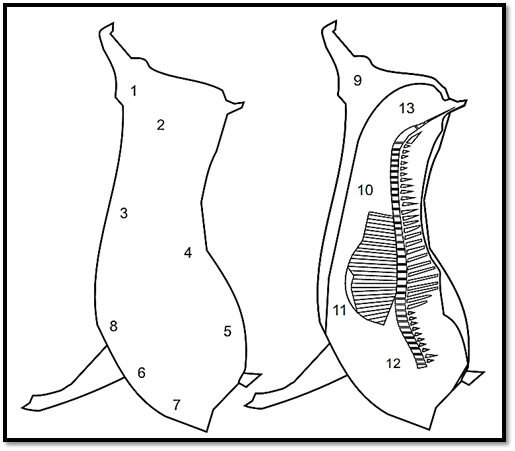
Figure 2 Points recommended by ISO 17604:2003/Amd.1:2009 for swab sampling of swine carcasses.
For analyses other than Salmonella the dilutions can be made directly from the BPW rinse. Alternatively, the carcass may be rinsed in Butterfield’s Phosphate Buffer instead of BPW. In this case, for Salmonella analysis add 30 ± 0.6 ml of double concentration BPW to 30 ± 0.6 ml of carcass-rinse fluid and mix well.
In this procedure each milliliter of washing liquid corresponds to the weight of the carcass divided by 400. For example, if the carcass weighs 1,600 g, each milliliter of the washing liquid corresponds to 4 g of the sample.
B- Procedure for washing other foods
Transfer the sample to a sterile bag and weigh. Using the same diluents recommended for swabs, add to the bag the amount of diluent required for an initial 1:1 dilution (1 ml of diluent per gram of sample). Closing the mouth of the bag with one hand, agitate the sample and massage the pieces inside the bag with the other hand from the outside, taking the necessary care and precautions to avoid that pointed or other protuberant parts come to pierce or puncture the package. In the case of grains, seeds, nuts, and similar products, the sample may also be placed in a flask containing the diluent and agitated for 10 min in a laboratory shaker. The liquid produced by this washing procedure may be used for both general quantification tests as for presence/absence tests which require enrichment in differ-entiated broth. In this procedure each milliliter of the washing liquid corresponds to 1 g of sample. The volume of diluent may vary, in accordance with the needs or the characteristics of the sample.
C- Procedure for washing packages
This procedure is recommended for packages with a leak-proof cap or closure system. In the case of packages that do not have any cap/closure system or caps that are not leak-proof, use the swabbing method. Using the same diluents as those recommended for swabs, add to the package an amount of diluent suf-ficient to wash the entire internal surface by agitation (1/5 of the package’s holding capacity, for example). Close the package tightly and, with the hands agitate and swirl the package vigorously to remove the micro-organisms adhered to the inner surface. Try to reach all the points of the inner surface, so as to guarantee complete removal of the contaminants present. The liquid obtained by this washing procedure may be used for both general quantification tests as for the presence/absence tests that require enrichment in differ-entiated broth (in the second case, follow the guidelines and instructions in each specific chapter). In this procedure each milliliter of the washing liquid corresponds to the holding capacity of the package divided by the volume of the diluent. For example, if the holding capacity of the package is 500 ml and the volume of diluent is equal to 100 ml, each milliliter of the washing liquid corresponds to 5 cm3.
Keeping of counter-samples
After withdrawing the analytical unit(s) store the remaining material under the same conditions utilized prior to analysis. Perishable samples need to be frozen, but it is important to know that thawing of counter-samples for the purpose of repeating microbiological test(s) is not an acceptable practice, due to the possible death of part of the microbial populations that were originally present. In the case of frozen products, this problem can be resolved by thawing for analysis only the portion required for the test(s). The remaining quantity, which was not thawed, may be kept frozen to be used as a counter-sample for later repetitions of the test(s), if necessary. In the case of refrigerated products, there is no acceptable way to keep counter-samples without freezing. In case test(s) need to be repeated, the result(s) should be interpreted taking into account the fact that population(s) of the target microorganism(s( may have been reduced due to freezing. In the case of samples the analytical unit of which has been collected by surface swab or sponge rubbing technique or the surface washing technique, the part of the diluent retaining the contaminants and not used for subsequent microbiological testing should be frozen to serve as counter-sample. Also in this case, it should be taken into consideration that the population(s) of the target microorganism(s) may have been reduced due to freezing.
The minimum time for keeping counter-samples is the time required for obtaining the results of the tests, but should be set at the discretion of the laboratory. The samples may be disposed of by throwing them in a dumpster, but samples deteriorated or suspected of containing microorganisms that are harmful to health should be decontaminated in an autoclave (121°C/30 min( prior to final disposal (ISO 7218:2007).
References
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R .(2013) . MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual . Institute of Food Technology – ITAL, Campinas, SP, Brazil.
Midura, T.F. & Bryant, R.G. (2001) Sampling plans, sample col-lection, shipment, and preparation for analysis. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 2, pp. 13–23.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


