

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Plate count method for coagulase positive staphylococci and S. aureus in foods
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
9-3-2016
5125
Plate count method for coagulase positive staphylococci and S. aureus in foods
Method of the American Public Health Association (APHA), as described in the 4th Edition of the Compendium of Methods for the Microbiological Examination of Foods (Lancette & Bennett, 2001) and the 17th Edition of the Standard Methods for the Examination of Dairy Products (Henning et al., 2004). This method is suitable for the analysis of foods in which more than 100 S. aureus cells/g may be expected.
1 - Material required for analysis
Preparation of the sample and serial dilutions
• Diluent: 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer
• Dilution tubes containing 9 ml of 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer
Direct plate count method
• Baird-Parker (BP) Agar plates
• Laboratory incubator set to 35–37°C (35 ± 1°C for dairy products)
Confirmation
• Brain Hearth Infusion Broth (BHI) tubes
• Trypticase Soy Agar (TSA) slants
• Coagulase plasma (rabbit) with EDTA
• Toluidine Blue-DNA agar plates or slides
• 3% Hydrogen Peroxide (for catalase test)
• 0.02M Phosphate-Saline Buffer
• Lysostaphin
• Purple Broth with 0.5% Glucose
• Purple Broth with 0.5% Mannitol
• Paraffin oil, sterile
• Gram Stain Reagents
• Laboratory incubator set to 35–37°C
10.2.2 Procedure
A general flowchart for the enumeration of coagulase positive staphylococci and Staphylococus aureus in foods using the plate count method APHA 2001 is shown in Figure 1.
a) Preparation of the samples and serial dilutions:
For dairy product analysis the Standard Methods for the Examination of Dairy Products recommends an analytical unit of 50 g.
b) Inoculation: Select three appropriate dilutions of the sample and inoculate 0.1 ml each on Baird Parker Agar, using the spread plate technique. Spread inoculum over surface of agar plate, using sterile bent glass streaking rod. Retain plates in upright position until inoculum is absorbed by agar.
Note b.1) For solid samples with low count, inoculate 1 ml of the first dilution and 0.1 ml of the two subsequent dilutions (distribute 1 ml of first dilution on four plates: 0.3 ml, 0.3 ml, 0.3 ml, and 0.1 ml). For liquid samples with low count, start with 1 ml of the sample without dilution.
c) Incubation and colony counting: Incubate plates (inverted) at 35–37°C/45–48 h.
Note c.1) The incubation temperature is 35 ± 1°C in the Standard Methods for the Examination of Dairy Products (Henning et al., 2004).
Examine plates for typical S. aureus colonies: black or gray, small (maximum 2–3 mm in diameter), surrounded by an opaque halo and frequently with an outer clear halo. Nonlipolytic strains form similar colonies but without the opaque and clear halos. Select plates containing 20–200 colonies for counting and if more than one type of presumptive S. aureus colonies are present count each type separately.
d) Confirmation: Select five typical colonies for coagulase test. If there are fewer than five colonies, select all. If several types of presumptive S.aureus colonies are present, select one or more colonies of each type.
Transfer suspect S. aureus colonies to Brain Heart Infusion (BHI) Broth tubes and emulsify. From the BHI tubes transfer a loopful to Trypticase Soy Agar (TSA) slants. Incubate BHI and TSA tubes at 35–37°C/18–24 h. Use the BHI culture for coagulase test. Keep TSA cultures at ambient temperature for other tests, if necessary.
d.1) Coagulase test: From the BHI culture transfer 0.2 ml to an empty sterile tube. Add 0.5 ml of reconstituted coagulase plasma with EDTA and mix. Use a tube with 0.2 ml of BHI and 0.5 ml of reconstituted coagulase plasma with EDTA as a negative control.
Incubate the tubes at 35–37°C in a water bath and examine periodically over a six-hour period for clot formation. Classify the result as described below.
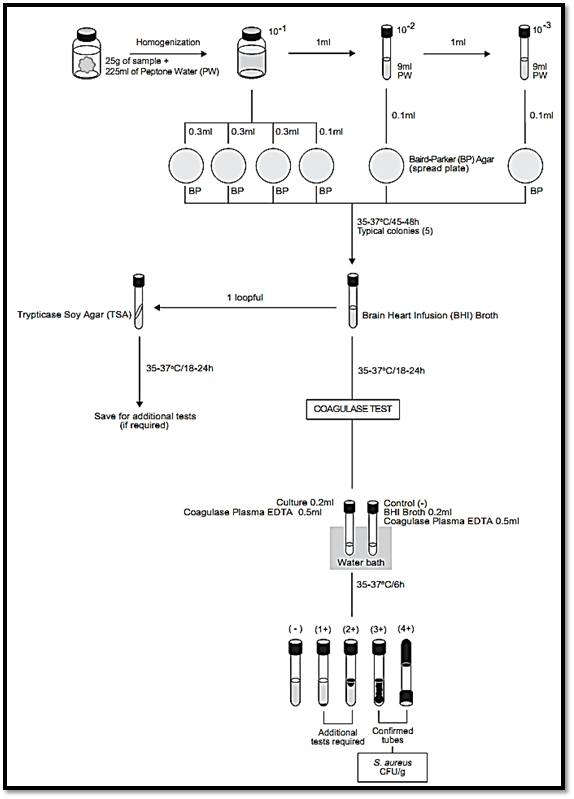
Figure.1 Scheme of analysis for the enumeration of coagulase positive staphylococci and Staphylococus aureus in foods using the plate count method APHA 2001 (Lancette & Bennett, 2001).
4+ positive: The entire content of tube coagulates and is not displaced when tube is inverted.
3+ positive: Large and organized clot.
2+ positive: Small organized clot.
1+ positive: Small unorganized clot.
Negative: No evidence of fibrin formation
A 3+ or 4+ clot formation is considered a positive reaction for S. aureus.
Note d.1.1) The Standard Methods for the Examination of Dairy Products (Henning et al., 2004) and the Bacteriological Analytical Manual (Bennett & Lancette, 2001) consider confirmed only cultures showing level 4+ positive coagulase test.
Note d.1.2.) A clumping factor latex agglutination test may be used if a more rapid procedure than coagulase test is desired. In this case, follow the manufacturers’ instructions.
Cultures showing levels 1+ or 2+ should be confirmed by performing the additional tests listed below. Observe the cultures for Gram stain and morphology. S. aureus are Gram positive cocci occurring typically in irregular clusters (resembling clusters of grapes).
d.2) Catalase test: From the TSA culture emulsify a loopful in one drop of 3% Hydrogen Peroxide on a glass slide. Immediate bubbling is a positive reaction. S. aureus is catalase positive.
d.3) Thermostable nuclease test ( thermonuclease test): Boil a portion of the culture grown in BHI for 15 min and use for thermonuclease test. Prepare microslides by spreading 3 ml Toluidine Blue DNA Agar on the surface of each microscope slide.
When agar has solidified, cut 2 mm or larger wells in agar (10–12 per slide) and fill with the boiled culture (about 0.01 ml). Also include S. aureus ATCC 12600 as a positive control and S. epidermidis ATCC 14990 as a negative control in the assay Incubate at 35–37°C/4 h or 50°C/2 h. Development of bright pink halo extending at least 1 mm from periphery of well indicates a positive reaction. Lack of the pink halo indicates a negative reaction. S. aureus is thermonuclease positive.
d.4) Lysostaphin sensitivity test: Mix 0.1 ml of the BHI culture with 0.1 ml of Lysostaphin (dissolved in 0.02 M Phosphate Buffer containing 2% NaCl) to give a final concentration of 25 μg lysostaphin/ml. To another 0.1 ml portion of the BHI culture, add 0.1 ml of 0.02 M Phosphate Buffer containing 2% NaCl (negative control). Also include S. aureus ATCC 12600 as a positive control and Kocuria varians ATCC 15306 (formerly Micrococcus varians) as a negative control in the assay. Incubate the tubes at 35°C for not more than 2 h. If turbidity clears in the test mixture, the test is considered positive (susceptible). No clearing after 2 h is considered a negative result (resistant). S. aureus is generally susceptible to lysostaphin, showing positive result in this test.
Note d.4.1) The Standard Methods for the Examination of Dairy Products (Henning et al., 2004) uses Lysostaphin dissolved in 0.02M Phosphate Buffer containing 1% NaCl.
d.5) Anaerobic utilization of glucose and mannitol. From the TSA slant, inoculate a tube of Purple Broth with 0.5% glucose and a tube of Purple Broth with 0.5% mannitol (exhaust oxygen from tubes before inoculation). Also include S. aureus ATCC 12600 as a positive control and Kocuria varians ATCC 15306 (formerly Micrococcus varians) as a negative control in the assay. Cover the surface of agar with a 25 mm layer of sterile paraffin oil. Incubate the tubes at 37°C for five days. The acid production anaerobically (color change to yellow throughout tube) indicates a positive test. Lack of change in color indicates a negative test. S .aureus is positive for anaerobic utilization of glucose.
Most strains are positive for mannitol, but some strains are negative. K. varians is negative for both.
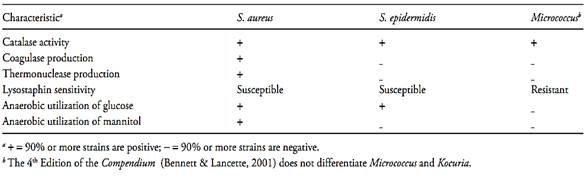
e) Interpretation and calculation of results: Consider as S. aureus the cultures showing coagulase test level 3+ and 4+ or cultures level 1+ and 2+ with typical characteristics presented in Table 1.
Calculate number of S. aureus cells/g of sample, based on percentage of colonies tested that are confirmed as S. aureus.
Example 1: The presumptive count obtained with 10−4 dilution of sample was 65 and four of five colonies tested were confirmed (80%). The number of S. aureus cells/g of food is 65 × 0.8 × 104 × 10 = 5.2 × 106 CFU/g (dilution factor is tenfold higher than sample dilution because only 0.1 ml was tested).
Table 1 Typical characteristics used to differentiate Staphylococcus aureus from Staphylococcus epidermidis and Micrococcus (Bennett & Lancette, 2001)
Example 2: The count obtained with 10−2 dilution of sample was 30 (20 colonies of one type and 10 colonies of another type). Five colonies of the first type tested were confirmed (100%). Two of five colonies of the second type tested were confirmed (40%). The number of S. aureus cells/g of food is (20 × 1 × 102 × 10) + (10 × 0.4 × 102 × 10) = 2.4 × 104 CFU/g .
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Henning, D.R., Flowers, R., Reiser, R. & Ryser, E.T. (2004) Patho-gens in milk and milk products. In: Wehr, H.M. & Frank, J.F (eds). Standard Methods for the Examination of Dairy Products. 17th edition. Washington, American Public Health Association. Chapter 5, pp. 103–151.
Lancette, G.A. & Bennett, R.W. (2001) Staphylococcus aureus and staphylococcal enterotoxins. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Associa-tion. Chapter 39, pp. 387–403.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















