

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Plate count method for Bacillus cereus in foods
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
9-3-2016
6183
Plate count method for Bacillus cereus in foods
Method of the American Public Health Association (APHA), as described the 4th Edition of the Compendium of Methods for the Microbiological Examination of Foods (Bennett & Belay, 2001).
1- Material required for analysis
Preparation of the sample and serial dilutions
• Diluent: 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer
• Dilution tubes containing 9 ml 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer
Direct plate count method
• Mannitol Egg Yolk Polymyxin (MYP) Agar plates or Kim-Goepfert (KG) Agar plates
• Laboratory incubator set to 30–32°C
B. cereus confirmation by Holbrook & Anderson test
• Spore Stain Reagents (Malachite Green Dye and Safranin Dye)
• Sudan Black B Solution (0.3% w/v in 70% ethanol)
• Xylene (reagent grade)
B. cereus confirmation by biochemical tests
• Mannitol Egg Yolk Polymyxin (MYP) Agar (plates) (if KG were used for the direct plating)
• Nutrient Agar (NA) (slants and plates)
• Phenol Red Carbohydrate Broth with 1% Glucose (tubes)
• Tyrosine Agar (tubes)
• Voges Proskauer (VP) Broth Modified for Bacillus (tubes)
• Nitrate Broth (tubes)
• Nutrient Broth with 0.001% lysozyme (tubes)
• Motility Medium for B. cereus
• Trypticase Soy Agar (TSA) with 5% Sheep Blood
• Anaerobic atmosphere generation system (Anaerogen from Oxoid, Anaerocult A from Merck, GasPak® from BD Biosciences, or equivalent)
• Voges-Proskauer (VP) Test Reagents (5% α-naphthol alcoholic solution, 40% potassium hydroxide aqueous solution, creatine phosphate crystals)
• Nitrate Test Reagents (sulfanilic acid solution, α-naphthol solution)
• Coomassie Brilliant Blue Solution
• Laboratory incubator set to 30°C
• Laboratory incubator set to 35°C
2 - Procedure
A general flowchart for the enumeration of Bacillus cereus in foods using the plate count method APHA 2001 is shown in Figure 1.
a) Preparation of the samples, inoculation, and incubation. Select three appropriate dilutions of the sample and inoculate 0.1 ml each on Mannitol Egg Yolk-Polymyxin (MYP) Agar or Kim-Goepfert (KG) Agar (using the spread plate technique). Incubate the plates (inverted) at 30–32°C/20–24 h.
Note a.1) For solid samples with low count, inoculate 1 ml of the first dilution and 0.1 ml of the two subsequent dilutions (distribute 1 ml of first dilution to four plates: 0.3 ml, 0.3 ml, 0.3 ml, and 0.1 ml). For liquid samples with low count, start with 1 ml of the sample without dilution.
Note a.2) If is necessary to count exclusively spores of B. cereus, apply a heat shock (70°C/15 min) to the sample.
b) Colony counting: Examine the plates for typical B. cereus colonies. The most commonly colonies seen on KG Agar are round, flat, dry, translucent or creamy white, surrounded by a wide precipitate zone of lecithinase activity. Less commonly the colonies may have irregular edges. The colonies on MYP are similar except that colonies and surrounding medium are pink (mannitol not fermented). Select for counting the plates containing 10 to 100 colonies.
Caution: MYP and KG allow the growth of B. anthracis and its colonies cannot be distinguished from B. cereus colonies. The plates and all the cultures isolated during confirmation should be handled with care.
c) B. cereus group confirmation: Select five or more presumptive positive colonies from the KG or MYP agar plates for confirmation. If there are fewer than five colonies, select all. Transfer each colony to nutrient agar slants and incubate at 30°C/24 h to inoculate the test media.
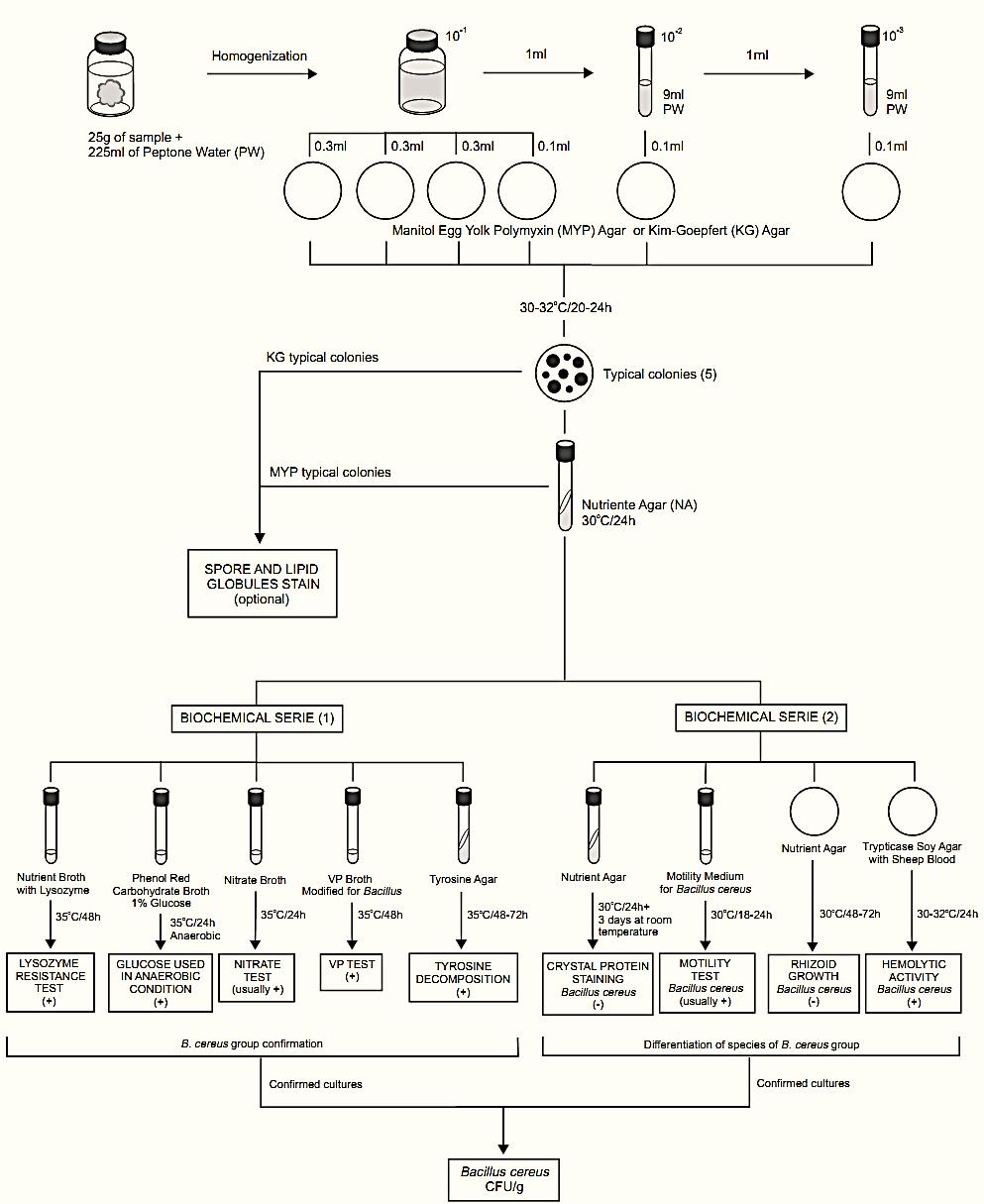
Figure 11.1 Scheme of analysis for the enumeration of Bacillus cereus in foods using the plate count method APHA 2001 (Bennett & Belay, 2001).
The Compendium of Methods for the Microbiological Examination of Foods (Bennett & Belay, 2001) suggests two ways to confirm the cultures as members of the B. cereus group, the biochemical tests of anaerobic glucose fermentation, tyrosine decomposition, Voges-Proskauer, nitrate reduction, and lysozyme resistance or the Holbrook & Anderson rapid confirmatory test (spore and lipid globules stain).
c.1) Anaerobic glucose fermentation: From each culture, inoculate a tube of Phenol Red Carbohydrate Broth with 1% glucose (exhaust oxygen from the tubes before inoculation). Incubate the tubes at 35°C/24 h in an anaerobic jar, using an anaerobic atmosphere generation systems (Anaerogen from Oxoid, Anaerocult A from Merck, GasPak® from BD Biosciences, or equivalent). A color change from red to yellow indicates a positive reaction (acid produced anaerobically).
The lack of the color change indicates a negative reaction. B. cereus and the other members of the B. cereus group ferment glucose anaerobically.
Note c.1) Use positive and negative control tubes because a partial color change from red to orange/yellow may occur even in non-inoculated tubes (pH reduction upon exposure of media to CO2 formed in the anaerobic jars).
c.2) Tyrosine decomposition: From each culture inoculate the surface of a Tyrosine Agar slant and incubate the tubes at 35°C/48–72 h. A positive test is indicated by a clearing zone immediately under the growth (tyrosine decomposed). B. cereus and the other members of the B. cereus group (except B. anthracis) decompose tyrosine.
c.3) Voges-Proskauer (VP) test: From each culture, inoculate a tube of Voges Proskauer (VP) Broth Modified for Bacillus and incubate the tubes at 35°C/48 h.To test for acetylmethylcarbinol, transfer 1 ml of culture to a test tube and add 0.2 ml of 40% KOH solution and 0.6 ml of 5% α-naphthol alcoholic solution. Shake, and add a few crystals of creatine. Observe results after 15 min at room temperature. Development of a pink or violet color indicates positive result. Lack of pink color indicates negative result. B. cereus and the other members of the B. cereus group are VP positive.
c.4) Nitrate reduction test: From each culture, inoculate a tube of Nitrate Broth and incubate the tubes at 35°C/24 h. To test for nitrate reduction to nitrite, add 0.25 ml each of nitrate test reagents ( sulfanilic acid solution and α-naphthol solution) to each culture. The development of an orange color within 10 min, indicates a positive reaction (nitrate reduced to nitrite). If no color develops (nitrite absent), test for residual nitrate by adding a small amount of zinc dust. An orange color indicates a negative reaction (nitrate is present, has not been reduced) and the absence of color indicates a positive reaction (nor nitrate or nitrite present, nitrate has been completely reduced to N2). B. cereus and the other members of the B. cereus group usually reduce nitrate to nitrite.
c.5) Lysozyme resistance: From each culture, inoculate a tube of Nutrient Broth (NB) with 0.001% lysozyme and a tube of NB without lysozyme (control). Incubate the tubes at 35°C/48 h and observe for growth in the presence of lysozyme (resistant strain) or only in NB without lysozyme (sensitive strain). B. cereus and the other members of the B. cereus group are resistant to lysozyme.
c.6) Holbrook & Anderson rapid confirmatory test ( spore and lipid globules stain): Colonies from KG may be tested directly from the KG plates. Colonies from MYP should be sub cultured on NA slants (30°C/24 h) before testing. Stain procedure:
• Prepare a smear from each colony.
• Place the slide over a boiling water and
flood with the Spore Stain Reagent Malachite Green Dye for two minutes. An acceptable alternative is heating the slide at least twice at 1 min interval with a Bunsen burner until steam is seen.
• After 2 min, wash the slide, blot dry, and stain for 20 min with a Sudan Black B Solution (0.3% w/v in 70% ethanol).
• Pour the stain off, blot dry the slide and wash with reagent grade xylene for 5–10s.
• Blot dry the slide immediately and counterstain for 20s with the Spore Stain Rea-gent Safranin Dye.
• Wash the slide, blot dry and examine microscopically under oil immersion. The members of the B. cereus group will show:
a) Lipid globules within the cytoplasm, stained dark blue. b) Central-to-subterminal spores that do not obviously swell the sporangium, stained pale to mid-green. c) Vegetative cells stained red.
d) Differentiating members of the B. cereus group: Presumptively identify as B. cereus those isolates which 1) produce large Gram-positive rods with spores that do not swell the sporangium; 2) pro-duce lecithinase and do not ferment mannitol on MYP agar; 3) grow and produce acid from glucose anaerobically; 4) reduce nitrate to nitrite (a few strains may be negative); 5) produce acetylmethylcarbinol (VP-positive); 6) decompose L-tyrosine; and 7) grow in the presence of 0.001% lysozyme. Use the tests described below to differentiate species within the B. cereus group.
d.1) Motility Test: From each culture, inoculate a tube of Motility Medium for B. cereus by stabbing. Incubate the tubes at 30°C/18–24 h. Motile strains grow away from the stab and non-motile strains growth only in and along the stab. Alternatively, the motility can be observed by microscope. Add 0.2 ml sterile distilled water to the surface of a Nutrient Agar (NA) slant and inoculate the slant with a loopful of the culture. Incubate the NA slants at 30°C/6–8 h and suspend a loopful of the liquid culture from the base of the slant in a drop of sterile water on a microscope slide. Apply a cover glass and examine microscopically under oil immersion. Most strains of B. cereus and B. thuringiensis are actively motile. B. anthracis and B. mycoides are nonmotile. A few B. cereus strains are also non-motile.
d.2) Rhizoid growth: From each culture, inoculate a pre-dried plate of Nutrient Agar (NA) by gently touching surface of medium near the center of each plate. Incubate the plates at 30°C/48–72 h and examine for development of rhizoid growth, which is characterized by production of colonies with root-like structures extending from the point of inoculation. This property is characteristic only of B. mycoides.
d.3) Hemolytic activity: Use one plate of Trypticase Soy Agar (TSA) with 5% Sheep Blood to inoculate eight cultures. Mark the bottom of the plate into eight sections and inoculate each section by gently touching the medium surface with a loopful of the culture. Incubate the plates at 30–32°C/24 h and examine for hemolytic activity, indicated by a clear zone of complete hemolysis surrounding the growth. B. cereus is hemolytic, B. thuringiensis and B. mycoides are often weakly hemolytic (produce hemolysis zone smaller than B. cereus or restricted to the region under the growth) and B. anthracis is usually nonhemolytic.
d.4) Crystal protein staining (method Sharif & Alaeddinoglu, 1988): Inoculate the culture onto a slant of Nutrient Agar (NA), incubate at 30°C/24 h and then held at room temperature for two or three days. Prepare a smear from the culture and heat fix with minimal flaming. Dip the slide into a small container containing a Coomassie Brilliant Blue Solution (coomassie brilliant blue 0.25 g + absolute ethanol 50 ml + glacial acetic acid 7 ml + water 43 ml) for 3 min. Wash the slide with tap water, dry and examine microscopically under oil immersion. B. thuringiensis will show free spores and toxin crystals. The released crystals can be distinguished from the spores since they stain purple and display a unique diamond (tetragonal) shape, while spores remain white and elliptical in appearance. Vegetative cells appear as purple rods. Crystals and spores appear as white bodies within purple stained cells. B. cereus does not produce crystals.
Note d.4.1) The procedure described in the Compendium is different, more laborious, and utilizes methanol – a toxic material that must be handled with care. Prepare a smear from the culture, fix with minimal flaming and further fix by flooding the slide with methanol. After 30s, pour off the methanol and dry the slide by passing it through a flame.
Flood the slide with 0.5% aqueous Basic Fuchsin Solution or TB Carbol Fuchsin ZN (Mycobacterium tuberculosis carbol fuchsin Ziehl-Neelsen stain) and gently heat the slide until steam is seen. After 1–2 min heat the slide again until steam is seen, held for 30s, and pour off the stain. Rinse the slide in water, dry and examine under oil immersion. The strains of B. thuringiensis will present free spores and a large quantity of red-stained, tetragonal-shaped crystals.
e) Calculation of results: Calculate number of B. cereus cells/g of sample, based on percentage of colonies tested that are confirmed as B. cereus.
Example: The presumptive count obtained with 10−4 dilution of sample was 65. Four of five colonies tested (80%) were confirmed as B. cereus. The number of B. cereus cells/g of food is (65 × 0.8 × 104 × 10) = 5.2 × 106 CFU/g (dilution factor is tenfold higher than sample dilution because only 0.1 ml was tested).
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Bennett, R.W. & Belay, N. (2001) Bacillus cereus. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 32, pp. 311–316.
Sharif, F.A. & Alaeddinoglu, N.G. (1988) A rapid and simple method for staining of the crystal protein of Bacillus thuringiensis. Journal of Industrial Microbiology, 3, 227–229.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















