
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-9-2018
Date: 12-7-2019
Date: 2-10-2020
|
Formation of a four-membered ring of carbon atoms can be achieved only with substantial distortion of the normal valence angles of carbon, regardless of whether the ring is planar or nonplanar. In cyclobutane, for example, if the valence bonds are assumed to lie along straight lines drawn between the carbon nuclei, each C−C−C bond angle will be 19.5o smaller than the 109.5o tetrahedral value:
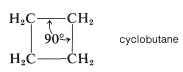
Figure 12-15).
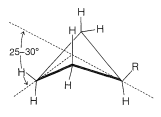
Figure 12-15: Nonplanar cyclobutane conformation with a substituent R in the less hindered, quasi-equatorial position. The dihedral angle between the two halves of the bent ring usually is 25o to 30o, that is, a 25o to 30o deviation from planarity.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
معهد الكفيل للنطق والتأهيل: أطلقنا برامج متنوعة لدعم الأطفال وتعزيز مهاراتهم التعليمية والاجتماعية
|
|
|