

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Catalyst Activity and Selectivity
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
21-1-2022
3710
Catalyst Activity and Selectivity
For maximum catalytic activity, the metal usually is prepared in a finely divided state. This is achieved for platinum and palladium by reducing the metal oxides with hydrogen prior to hydrogenation of the alkene. A specially active form of nickel ("Raney nickel") is prepared from a nickel-aluminum alloy. Sodium hydroxide is added to the alloy to dissolve the aluminum. The nickel remains as a black powder which is pyrophoric (burns in air) if not kept moist:
Highly active platinum, palladium, and nickel catalysts also can be obtained by reduction of metal salts with sodium borohydride (NaBH4).
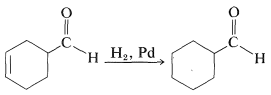
As mentioned previously, multiple bonds are not hydrogenated with equal facility. This fact can be used to advantage in carrying out selective reactions. For instance, hydrogenation of a carbon-carbon double bond can be achieved without simultaneously reducing a carbonyl bond in the same molecule. For example the carbon-carbon double bond of the following aldehyde can be reduced selectively:
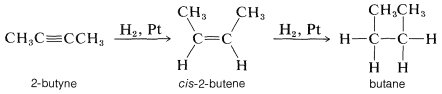
Alkynes are hydrogenated more easily than alkenes mainly because alkynes are adsorbed more readily on the catalyst surface. Hydrogenation proceeds in stages, first to the cis-alkene and then to the alkane. For example,
Normally, it is not possible to stop the hydrogenation of an alkyne at the alkene stage, but if the catalyst is suitably deactivated, addition to the triple bond can be achieved without further addition occurring to the resulting double bond. The preferred catalyst for selective hydrogenation of alkynes is palladium partially "poisoned" with a lead salt (Lindlar catalyst). This catalyst shows little affinity for adsorbing alkenes and hence is ineffective in bringing about hydrogenation to the alkane stage:
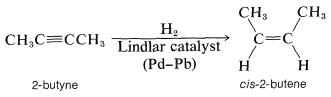
Aromatic hydrocarbons are hydrogenated with considerable difficulty, requiring higher temperatures, higher pressures, and longer reaction times than for alkenes or alkynes:
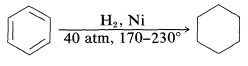
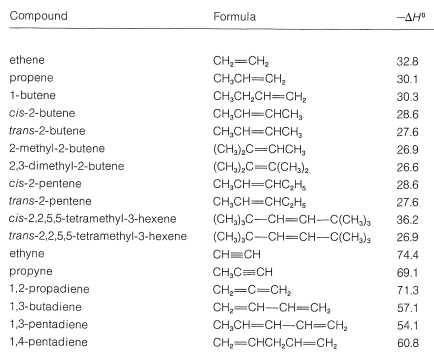
In addition to having synthetic applications, catalytic hydrogenation is useful for analytical and thermochemical purposes. The analysis of a substance for the number of carbon-carbon double bonds it contains is carried out by measuring the uptake of hydrogen for a known amount of sample. Measurement of the heat evolved in the hydrogenation of alkenes gives information as to the relative stabilities of alkenes, provided that the differences in ΔS0 values are small.
The experimental values of ΔH0 for hydrogenation of a number of alkenes and alkynes are listed in Table 11-2. The ΔH0 calculated from average bond energies is −30kcal/mol for a double bond and −69kcal/mol for a triple bond. The divergences from these values reflect the influence of structure on the strengths of multiple bonds. Some important generalizations can be made:
- The more alkyl groups or other substituents there are on the multiple bond, the less heat is evolved on hydrogenation. Because less heat evolved signifies a stronger, more stable bond, it appears that alkyl substitution increases the stability (strength) of the multiple bond.
- Trans isomers of 1,2-dialkyl-substituted ethenes evolve less heat (are more stable) than the corresponding cis isomers. This is the result of molecular overcrowding in the cis isomers from nonbonded interactions between two alkyl groups on the same side of the double bond. The effect amounts to almost 10kcal/mol with two cis-tert-butyl groups. This effect is another manifestation of steric hindrance and can be seen most clearly with space-filling models (Figure 11-3).
Figure 11-3: Space-filling models of cis- and trans-2,2,5,5-tetramethyl-3-hexene, which show the difference in steric interactions between the tert-butyl groups.
3. Conjugated dienes are more stable than isolated dienes (compare 1,3- and 1,4-pentadiene).
4. Cumulated dienes appear to be less stable than conjugated or isolated dienes (see 1,2-propadiene).
Table 11-2: Heats of Hydrogenation of Gaseous Alkenes and Alkynes (kcal/mol,1atm,25o)
 الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















