Orientation of Addition
The direction of addition of hydrogen bromide to propene clearly depends on which end of the double bond the bromine atom attacks. The important question is which of the two possible carbon radicals that may be formed is the more stable, the 1-bromo-2-propyl radical, 5, or the 2-bromo-1-propyl radical, 6:
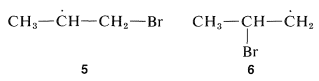
From C−H bond-dissociation energies of alkanes , the ease of formation and stabilities of the carbon radicals is seen to follow the sequence tertiary >> secondary >> primary. By analogy, the secondary 1-bromo-2-propyl radical, 5, is expected to be more stable and more easily formed than the primary 2-bromo-1-propyl radical, 66. The product of radical addition should be, and indeed is, 1-bromopropane:
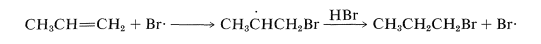
Other reagents, such as the halogens, also can add to alkenes and alkynes by both radical-chain and ionic mechanisms. Radical addition usually is initiated by light, whereas ionic addition is favored by low temperatures and no light. Nevertheless, it often is difficult to keep both mechanisms from operating at the same time. This is important even when the alkene is symmetrical because, although the adduct will then have the same structural formula regardless of mechanism, the stereochemical configurations may differ. Electrophilic addition of halogens generally is a stereospecific antarafacial addition, but radical-chain additions are less stereospecific.
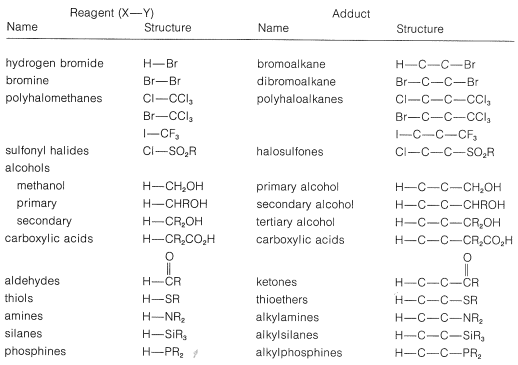
There are many reagents that add to alkenes only by radical-chain mechanisms. A number of these are listed in Table 10-3. They have in common a relatively weak bond, X−Y, that can be cleaved homolytically either by light or by chemical initiators such as peroxides. In the propagation steps, the radical that attacks the double bond does so to produce the more stable carbon radical. For addition to simple alkenes and alkynes, the more stable carbon radical is the one with the fewest hydrogens or the most alkyl groups at the radical center.
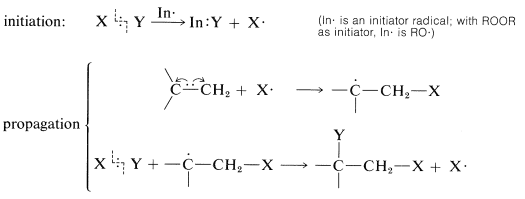
Table 10-3: Reagents that add to Alkenes by Radical-Chain Mechanisms
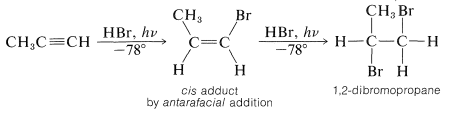
The principles of radical addition reactions of alkenes appear to apply equally to alkynes, although there are fewer documented examples of radical additions to triple bonds. Two molecules of hydrogen bromide can add to propyne first to give cis-1-bromopropene (by antarafacial addition) and then 1,2-dibromopropane: