
Electronegativity and Chemical Shifts
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 13-1-2022
13-1-2022
 2809
2809
Electronegativity and Chemical Shifts
Consider first the chemical shifts of protons attached to an sp3 carbon, 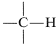 .
.
The degree of shielding of the proton by the carbon valence electrons depends on the character of the substituent atoms and groups present, and particularly on their electron-attracting power, or electronegativity. For a grouping of the type 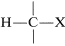 , the shielding will be less as X is more electron withdrawing relative to hydrogen:
, the shielding will be less as X is more electron withdrawing relative to hydrogen:
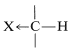 If X is electron-withdrawing, the proton is deshielded.
If X is electron-withdrawing, the proton is deshielded.
For example, the proton chemical shifts of the methyl halides (Table 9-4) show decreasing shielding, hence progressively low-field chemical shifts with increasing halogen electronegativity (F>Cl>Br>I):

Figure 9-28 shows how the shift differences between the CH3− and the −CH2− protons in some CH3CH2X derivatives depend on the electronegativity of X, using the electronegativity defined by L. Pauling. The trend is not wholly linear, but the proton chemical-shift differences become larger the more electronegative X becomes. We can predict with some confidence, therefore, that a molecule such as XCH2CH2Y will have lower-field chemical shifts (larger δ) for XCH2− than for −CH2Y if X is more electronegative than Y:
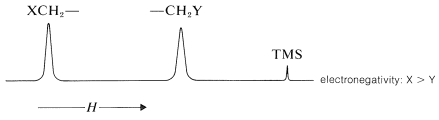
Table 9-4: Typical Proton Chemical-Shift Values (δ) in Dilute CHCl3 Solutions
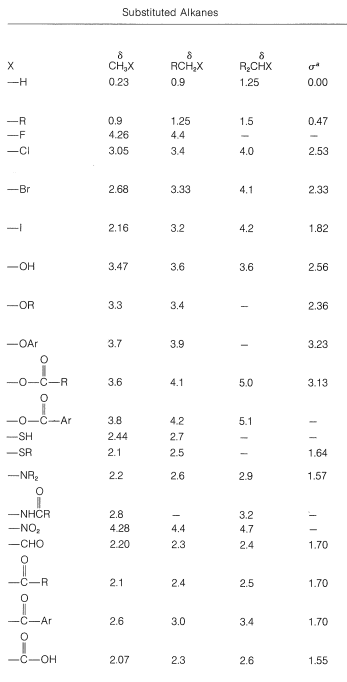

Figure 9-28: Chemical-shift differences between the CH3 and CH2 protons of CH3CH2X derivatives as a function of the Pauling electronegativity of X.
When two electronegative groups, X and Y, are bonded to the same carbon, as in XCH2YXCH2Y, the protons are expected to be less shielded and come into resonance downfield of the methylenes of XCH2CH2Y. There is an approximate relationship (see below) between the shifts of the XCH2Y protons and the effective shielding constants (σ) of X and Y known as Shoolery's rule.
δ=0.23+σx+σy (9-4)
Appropriate values of σσ for use with this equation are given in Table 9-4.
 الاكثر قراءة في التشخيص العضوي
الاكثر قراءة في التشخيص العضوي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


