

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Purine Nucleotide Degradation
المؤلف:
Denise R. Ferrier
المصدر:
Lippincott Illustrated Reviews: Biochemistry
الجزء والصفحة:
17-11-2021
3422
Purine Nucleotide Degradation
Degradation of dietary nucleic acids occurs in the small intestine, where pancreatic nucleases hydrolyze them to nucleotides. The nucleotides are sequentially degraded by intestinal enzymes to nucleosides, phosphorylated sugars, and free bases. Uric acid is the end product of intestinal purine degradation. [Note: Purine nucleotides from de novo synthesis are degraded in the liver primarily. The free bases are sent out from the liver and salvaged by peripheral tissues.]
A. Degradation in the small intestine
Ribonucleases and deoxyribonucleases, secreted by the pancreas, hydrolyze dietary RNA and DNA to oligonucleotides that are further hydrolyzed by pancreatic phosphodiesterases, producing a mixture of 3′-and 5′-mononucleotides. At the intestinal mucosal surface, nucleotidases remove the phosphate groups hydrolytically, releasing nucleosides that are taken into enterocytes by sodium-dependent transporters and degraded by nucleosidases (nucleoside phosphorylases) to free bases plus (deoxy) ribose 1-phosphate. Dietary purine bases are not used to any appreciable extent for the synthesis of tissue nucleic acids. Instead, they are degraded to uric acid in the enterocytes. Most of the uric acid enters the blood and is eventually excreted in the urine. A summary of this pathway is shown in Figure 1. [Note: Mammals other than primates express urate oxidase (uricase), which cleaves the purine ring, generating allantoin. Modified recombinant urate oxidase is now used clinically to lower urate levels.]
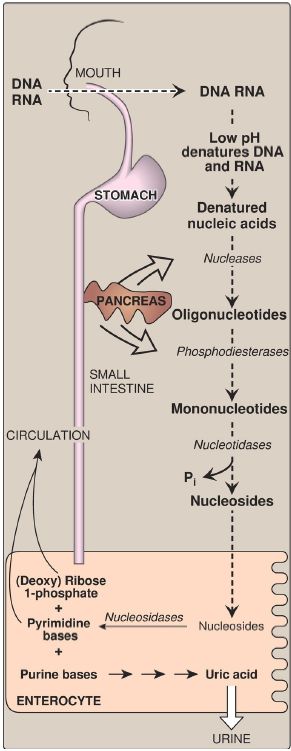
Figure 1: Digestion of dietary nucleic acids. Pi = inorganic phosphate.
B. Uric acid formation
A summary of the steps in the production of uric acid and the genetic diseases associated with deficiencies of specific degradative enzymes are shown in Figure 2. [Note: The bracketed numbers refer to specific reactions in the figure.]
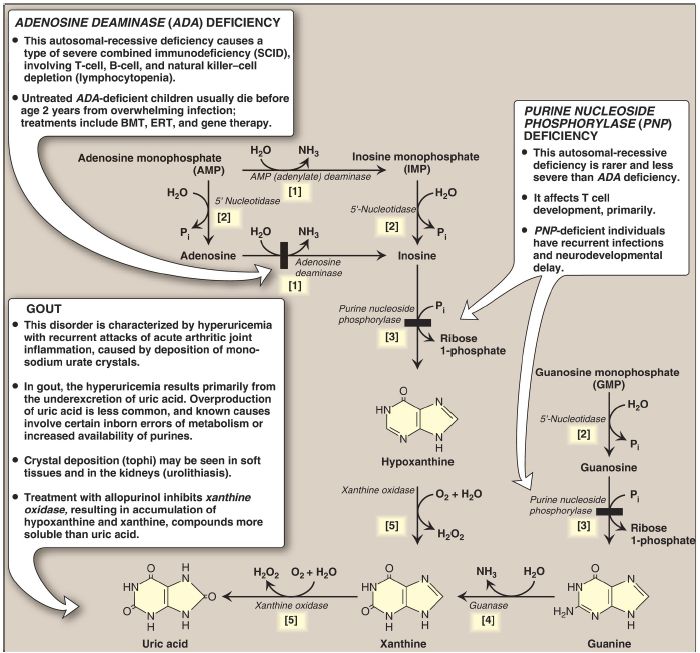
Figure 2: The degradation of purine nucleotides to uric acid, illustrating some of the genetic diseases associated with this pathway. [Note: The numbers in brackets refer to the corresponding numbered citations in the text.] BMT = bone marrow transplantation; ERT = enzyme replacement therapy; Pi = inorganic phosphate; H2O2 = hydrogen peroxide; NH3 = ammonia.
[1] An amino group is removed from AMP to produce IMP by AMP (adenylate) deaminase or from adenosine to produce inosine (hypoxanthine-ribose) by adenosine deaminase.
[2] IMP and GMP are converted into their respective nucleoside forms, inosine and guanosine, by the action of 5′-nucleotidase.
[3] Purine nucleoside phosphorylase converts inosine and guanosine into their respective purine bases, hypoxanthine and guanine. [Note: A mutase interconverts ribose 1- and ribose 5-phosphate.]
[4] Guanine is deaminated to form xanthine.
[5] Hypoxanthine is oxidized by molybdenum-containing xanthine oxidase (XO) to xanthine, which is further oxidized by XO to uric acid, the final product of human purine degradation. Uric acid is excreted primarily in the urine.
C. Diseases associated with purine degradation
1. Gout: Gout is a disorder initiated by high levels of uric acid (the end product of purine catabolism) in blood (hyperuricemia), as a result of either the overproduction or underexcretion of uric acid. The hyperuricemia can lead to the deposition of monosodium urate (MSU) crystals in the joints and an inflammatory response to the crystals, causing first acute and then progressing to chronic gouty arthritis.
Nodular masses of MSU crystals (tophi) may be deposited in the soft tissues, resulting in chronic tophaceous gout (Fig. 3). Formation of uric acid stones in the kidney (urolithiasis) may also be seen. [Note: Hyperuricemia is not sufficient to cause gout, but gout is always preceded by hyperuricemia. Hyperuricemia is typically asymptomatic but may be indicative of comorbid conditions such as hypertension.] The definitive diagnosis of gout requires aspiration and examination of synovial fluid (Fig. 4) from an affected joint (or material from a tophus) using polarized light microscopy to confirm the presence of needle-shaped MSU crystals (Fig. 5).
Figure 3:Tophaceous gout.
Figure 4: Analysis of joint fluid can help to define causes of joint swelling and arthritis, such as infection, gout, and rheumatoid disease.
Figure 5: Gout can be diagnosed by the presence of negatively birefringent monosodium urate crystals in aspirated synovial fluid examined by polarized light microscopy. Here, crystals are seen within polymorphonuclear leukocytes.
a. Uric acid underexcretion: In >90% of individuals with hyperuricemia, the cause is underexcretion of uric acid. Underexcretion can be primary, because of as-yet-unidentified inherent excretory defects, or secondary to known disease processes that affect how the kidney handles urate (for example, in lactic acidosis, lactate increases renal urate reabsorption, thereby decreasing its excretion) and to environmental factors such as the use of drugs (for example, thiazide diuretics) or exposure to lead (saturnine gout).
b. Uric acid overproduction: A less common cause of hyperuricemia is from the overproduction of uric acid. Primary hyperuricemia is, for the most part, idiopathic (having no known cause). However, several identified mutations in the gene for X-linked PRPP synthetase result in the enzyme having an increased maximal velocity ([Vmax] ) for the production of PRPP, a lower Km for ribose 5-phosphate, or a decreased sensitivity to purine nucleotides, its allosteric inhibitors . In each case, increased availability of PRPP increases purine production, resulting in elevated levels of plasma uric acid. Lesch-Nyhan syndrome also causes hyperuricemia as a result of the decreased salvage of hypoxanthine and guanine and the subsequent increased availability of PRPP. Secondary hyperuricemia is typically the consequence of increased availability of purines (for example, in patients with myeloproliferative disorders or who are undergoing chemotherapy and so have a high rate of cell turnover). Hyperuricemia can also be the result of seemingly unrelated metabolic diseases, such as von Gierke disease or hereditary fructose intolerance ).
A diet rich in meat, seafood (particularly shellfish), and ethanol is associated with increased risk of gout, whereas a diet rich in low-fat dairy products is associated with a decreased risk.
c. Treatment: Acute attacks of gout are treated with anti-inflammatory agents. Colchicine, steroidal drugs such as prednisone, and nonsteroidal drugs such as indomethacin are used. [Note: Colchicine prevents formation of microtubules, thereby decreasing the movement of neutrophils into the affected area. Like the other anti-inflammatory drugs, it has no effect on uric acid levels.] Long-term therapeutic strategies for gout involve lowering the uric acid level below its saturation point (6.5 mg/dl), thereby preventing the deposition of MSU crystals. Uricosuric agents, such as probenecid or sulfinpyrazone, that increase renal excretion of uric acid, are used in patients who are underexcretors of uric acid. Allopurinol, a structural analog of hypoxanthine, inhibits uric acid synthesis and is used in patients who are overproducers of uric acid. Allopurinol is oxidized to oxypurinol, a long-lived inhibitor of XO. This results in an accumulation of hypoxanthine and xanthine (see Fig. 2), compounds more soluble than uric acid and, therefore, less likely to initiate an inflammatory response. In patients with normal levels of HGPRT, the hypoxanthine can be salvaged, reducing the levels of PRPP and, therefore, de novo purine synthesis. Febuxostat, a nonpurine inhibitor of XO, is also available. [Note: Uric acid levels in the blood normally are close to the saturation point. One reason for this may be the strong antioxidant effects of uric acid.]
2. Adenosine deaminase deficiency
ADA is expressed in a variety of tissues, but, in humans, lymphocytes have the highest activity of this cytoplasmic enzyme. A deficiency of ADA results in an accumulation of adenosine, which is converted to its ribonucleotide or deoxyribonucleotide forms by cellular kinases. As dATP levels rise, ribonucleotide reductase is inhibited, thereby preventing the production of all deoxyribose-containing nucleotides . Consequently, cells cannot make DNA and divide. [Note: The dATP and adenosine that accumulate in ADA deficiency lead to developmental arrest and apoptosis of lymphocytes.] In its most severe form, this autosomal-recessive disorder causes a type of severe combined immunodeficiency disease (SCID), involving a decrease in T cells, B cells, and natural killer cells. ADA deficiency accounts for ~14% of cases of SCID in the United States. Treatments include bone marrow transplantation, enzyme replacement therapy, and gene therapy . Without appropriate treatment, children with this disorder usually die from infection by age 2 years. [Note: Purine nucleoside phosphorylase deficiency results in a less severe immunodeficiency primarily involving T cells.]
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















