

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Eukaryotic Fatty Acid Synthase
المؤلف:
Denise R. Ferrier
المصدر:
Lippincott Illustrated Reviews: Biochemistry
الجزء والصفحة:
8-10-2021
2356
Eukaryotic Fatty Acid Synthase
The remaining series of reactions of fatty acid synthesis in eukaryotes is catalyzed by the multifunctional, homodimeric enzyme fatty acid synthase (FAS). The process involves the addition of two carbons from malonyl CoA to the carboxyl end of a series of acyl acceptors.
Each FAS monomer is a multicatalytic polypeptide with six different enzymic domains plus a 4ʹ- phosphopantetheine-containing acyl carrier protein (ACP) domain. 4ʹ- Phosphopantetheine, a derivative of pantothenic acid (vitamin B5), carries acyl units on its terminal thiol (–SH) group and presents them to the catalytic domains of FAS during fatty acid synthesis. It also is a component of CoA. [Note: In prokaryotes, FAS is a multienzyme complex.] The reaction numbers in brackets below refer to Figure 1.
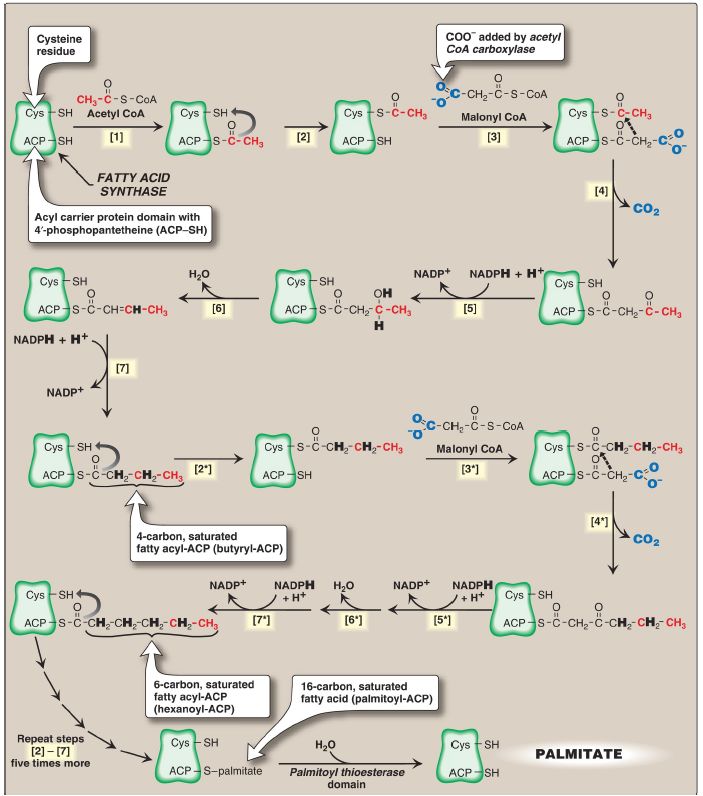
Figure 1: Synthesis of palmitate (16:0) by multifunctional fatty acid synthase. [Note: Numbers in brackets correspond to bracketed numbers in the text. A second repetition of the steps is indicated by numbers with an asterisk (*). Carbons provided directly by acetyl coenzyme A (CoA) are shown in red.] ACP = acyl carrier protein domain; CO2 = carbon dioxide; NADP(H) = nicotinamide adenine dinucleotide phosphate.
1. An acetyl group is transferred from acetyl CoA to the –SH group of the ACP. Domain: Malonyl/acetyl CoA–ACP transacylase.
2. Next, this two-carbon fragment is transferred to a temporary holding site, the –SH group of a cysteine residue on the condensing enzyme domain (see [4] below).
3. The now-vacant ACP accepts a three-carbon malonyl group from malonyl CoA. Domain: Malonyl/acetyl CoA–ACP transacylase.
4. The acetyl group on the cysteine residue condenses with the malonyl group on ACP as the CO2 originally added by ACC is released. The result is a four-carbon unit attached to the ACP domain. The loss of free energy from the decarboxylation drives the reaction. Domain: 3- Ketoacyl–ACP synthase, also known as condensing enzyme.
The next three reactions convert the 3-ketoacyl group to the corresponding saturated acyl group by a pair of NADPH-requiring reductions and a dehydration step.
1. The keto group is reduced to an alcohol. Domain: 3-Ketoacyl–ACP reductase.
2. A molecule of water is removed, creating a trans double bond between carbons 2 and 3 (the α- and β-carbons). Domain: 3-Hydroxyacyl–ACP dehydratase.
3. The double bond is reduced. Domain: Enoyl–ACP reductase.
This sequence of steps results in the production of a four-carbon group (butyryl) whose three terminal carbons are fully saturated and which remains attached to the ACP domain. The steps are repeated (indicated by an asterisk), beginning with the transfer of the butyryl unit from the ACP to the cysteine residue [2*], the attachment of a malonyl group to the ACP [3*], and the condensation of the two groups liberating CO2 [4*]. The carbonyl group at the β-carbon (carbon 3, the third carbon from the sulfur) is then reduced [5*], dehydrated [6*], and reduced [7*], generating hexanoyl-ACP. This cycle of reactions is repeated five more times, each time incorporating a two-carbon unit (derived from malonyl CoA) into the growing fatty acid chain at the carboxyl end. When the fatty acid reaches a length of 16 carbons, the synthetic process is terminated with palmitoyl-S-ACP. [Note: Shorter-length fatty acids are produced in the lactating mammary gland.] Palmitoyl thioesterase, the final catalytic activity of FAS, cleaves the thioester bond, releasing a fully saturated molecule of palmitate (16:0). [Note: All the carbons in palmitic acid have passed through malonyl CoA except the two donated by the original acetyl CoA (the first acyl acceptor), which are found at the methyl (ω) end of the fatty acid. This underscores the rate-limiting nature of the ACC reaction.]
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















