

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Chemiosmotic hypothesis
المؤلف:
Denise R. Ferrier
المصدر:
Lippincott Illustrated Reviews: Biochemistry
الجزء والصفحة:
11-9-2021
2315
Chemiosmotic hypothesis
The chemiosmotic hypothesis (also known as the Mitchell hypothesis) explains how the free energy generated by the transport of electrons by the ETC is used to produce ATP from ADP + Pi.
1. Proton pump: Electron transport is coupled to ADP phosphorylation by the pumping of H+ across the inner mitochondrial membrane, from the matrix to the intermembrane space, at Complexes I, III, and IV. For each pair of electrons transferred from NADH to O2, 10 H+ are pumped. This creates an electrical gradient (with more positive charges on the cytosolic side of the membrane than on the matrix side) and a pH (chemical) gradient (the cytosolic side of the membrane is at a lower pH than the matrix side), as shown in Figure 1. The energy (proton-motive force) generated by these gradients is sufficient to drive ATP synthesis. Thus, the H+ gradient serves as the common intermediate that couples oxidation to phosphorylation.
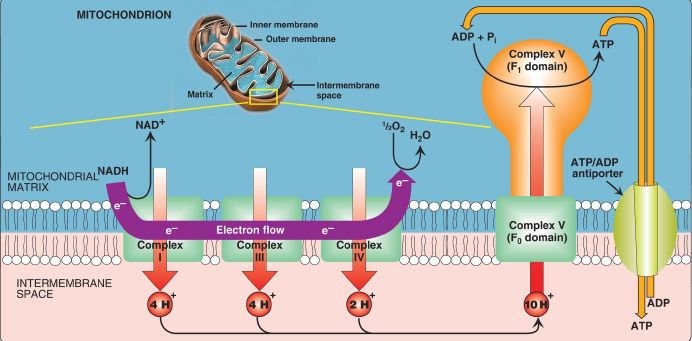
Figure 1: Electron transport chain shown in association with proton (H+) pumping. Ten H+ are pumped for each nicotinamide adenine dinucleotide (NADH) oxidized. [Note: H+ are not pumped at Complex II.] e− = electron; Complex V = ATP synthase.
2. ATP synthase: The multisubunit enzyme ATP synthase ([Complex V] Fig. 2) synthesizes ATP using the energy of the H+ gradient. It contains a membrane domain (Fo) that spans the inner mitochondrial membrane and an extramembranous domain (F1) that appears as a sphere that protrudes into the mitochondrial matrix (see Fig. 1). The chemiosmotic hypothesis proposes that after H+ have been pumped to the cytosolic side of the inner mitochondrial membrane, they reenter the matrix by passing through a H+ channel in the Fo domain, driving rotation of the c ring of Fo and, at the same time, dissipating the pH and electrical gradients. Rotation in Fo causes conformational changes in the three β subunits of F1 that allow them to bind ADP + Pi, phosphorylate ADP to ATP, and release ATP. One complete rotation of the c ring produces three ATP. [Note: ATP synthase is also called F1/Fo-ATPase because the enzyme can also catalyze the hydrolysis of ATP to ADP and Pi.]
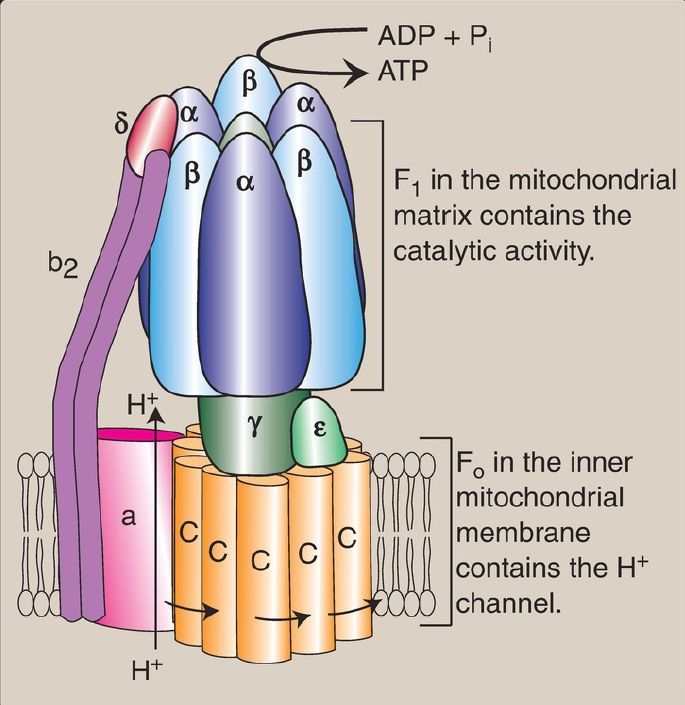
Figure 2: ATP synthase (F1Fo-ATPase). [Note: The c ring of vertebrates contains eight subunits. One complete turn of the ring is driven by eight H+ (protons) moving through the Fo domain. The resulting conformational changes in the three β subunits of the F1 domain allow phosphorylation of three adenosine diphosphates (ADP) to three ATP.] Pi = inorganic phosphate.
a. Coupling in oxidative phosphorylation: In normal mitochondria, ATP synthesis is coupled to electron transport through the H+ gradient. Increasing (or decreasing) one process has the same effect on the other. For example, hydrolysis of ATP to ADP and Pi in energyrequiring reactions increases the availability of substrates for ATP synthase and, thus, increases H+ flow through the enzyme. Electron transport and H+ pumping by the ETC increase to maintain the H+ gradient and allow ATP synthesis.
b. Oligomycin: This drug binds to the Fo (hence the letter “o”) domain of ATP synthase, closing the H+ channel and preventing reentry of H+ into the matrix, thereby inhibiting phosphorylation of ADP to ATP.
Because the pH and electrical gradients cannot be dissipated in the presence of this phosphorylation inhibitor, electron transport stops because of the difficulty of pumping any more H+ against the steep gradient. This dependency of cellular respiration on the ability to phosphorylate ADP to ATP is known as respiratory control and is the consequence of the tight coupling of these processes.
c. Uncoupling proteins: Uncoupling proteins (UCP) occur in the inner mitochondrial membrane of mammals, including humans. These proteins form channels that allow H+ to reenter the mitochondrial matrix without energy being captured as ATP (Fig. 3). The energy is released as heat, and the process is called nonshivering thermogenesis. UCP1, also called thermogenin, is responsible for heat production in the mitochondria-rich brown adipocytes of mammals. [Note: Cold causes catecholamine-dependent activation of UCP1 expression.] In brown fat, unlike the more abundant white fat, ~90% of its respiratory energy is used for thermogenesis in infants in response to cold. Thus, brown fat is involved in energy expenditure, whereas white fat is involved in energy storage. [Note: Brown fat depots have recently been shown to be present in adults.]
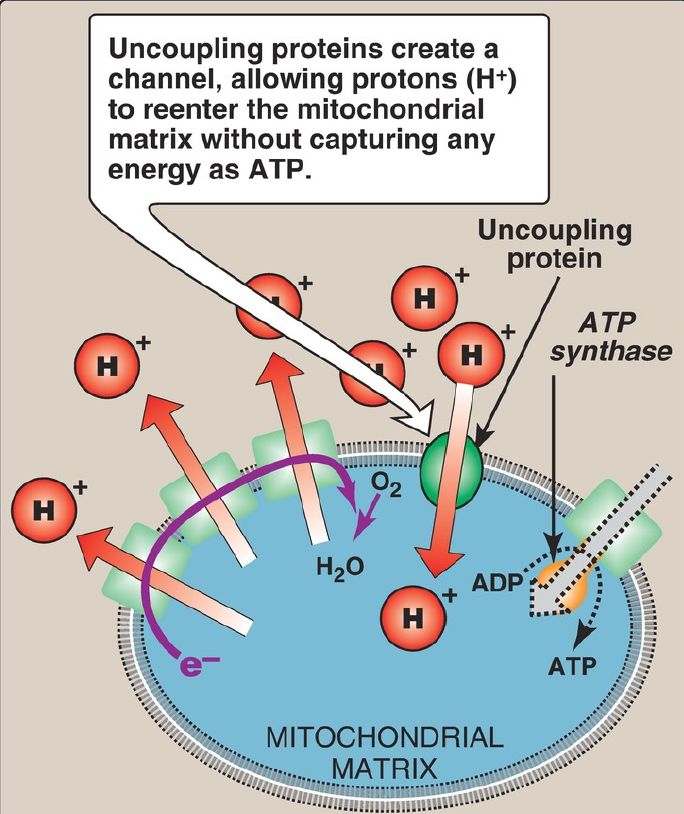
Figure 3: Transport of protons across the mitochondrial membrane by an uncoupling protein. ADP = adenosine diphosphate; e− = electrons.
d. Synthetic uncouplers: Electron transport and phosphorylation of ADP can also be uncoupled by compounds that shuttle H+ across the inner mitochondrial membrane, dissipating the gradient. The classic example is 2,4-dinitrophenol, a lipophilic H+ carrier (ionophore) that readily diffuses through the mitochondrial membrane (Fig. 4). This uncoupler causes electron transport to proceed at a rapid rate without establishing a H+ gradient, much as do the UCP. Again, energy is released as heat rather than being used to synthesize ATP. [Note: In high doses, aspirin and other salicylates uncouple oxidative phosphorylation. This explains the fever that accompanies toxic overdoses of these drugs.]
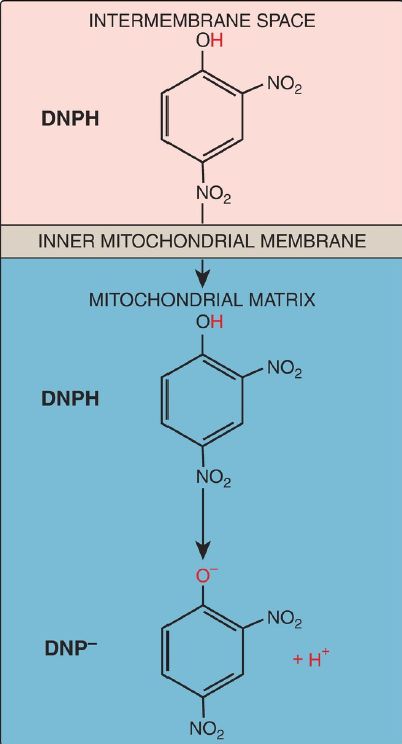
Figure 4: 2,4-Dinitrophenol (DNP), a proton (H+) carrier, shown in its reduced (DNPH) and oxidized (DNP–) forms.
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















