
The DNA-Binding Form of the Lambda Repressor Is a Dimer
 المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
 المصدر:
LEWIN’S GENES XII
المصدر:
LEWIN’S GENES XII
 الجزء والصفحة:
الجزء والصفحة:
 7-6-2021
7-6-2021
 2217
2217
The DNA-Binding Form of the Lambda Repressor Is a Dimer
KEY CONCEPTS
- A repressor monomer has two distinct domains.
- The N-terminal domain contains the DNA-binding site.
- The C-terminal domain dimerizes.
- Binding to the operator requires the dimeric form so that two DNA-binding domains can contact the operator simultaneously.
- Cleavage of the repressor between the two domains reduces the affinity for the operator and induces a lytic cycle.
The lambda repressor subunit is a polypeptide of 27 kD with the two distinct domains shown in FIGURE 1:
- The N-terminal domain, residues 1–92, provides the operatorbinding site.
- The C-terminal domain, residues 132–236, is responsible for dimerization.
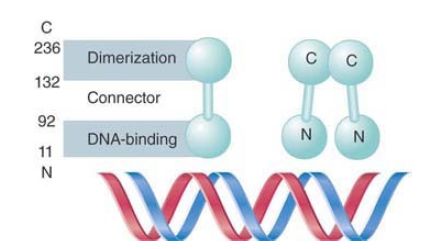
FIGURE 1. The N-terminal and C-terminal regions of repressor form separate domains. The C-terminal domains associate to form dimers; the N-terminal domains bind DNA.
The two domains are joined by a connector of 40 residues. When repressor is digested by a protease, each domain is released as a separate fragment.
Each domain can exercise its function independently of the other. The C-terminal fragment can form oligomers. The N-terminal fragment can bind the operators, though with a lower affinity than the intact lambda repressor. Thus, the information for specifically contacting DNA is contained within the N-terminal domain, but the efficiency of the process is enhanced by the attachment of the Cterminal domain.
The dimeric structure of the lambda repressor is crucial in maintaining lysogeny. The induction of a lysogenic prophage into the lytic cycle is caused by cleavage of the repressor subunit in the connector region, between residues 111 and 113. (This is a counterpart to the allosteric change in conformation that results when a small-molecule inducer inactivates the repressor of a bacterial operon, a capacity that the lysogenic repressor does not have.) Induction occurs under certain adverse conditions, such as exposure of lysogenic bacteria to ultraviolet (UV) irradiation, which leads to proteolytic inactivation of the repressor due to the induction of the SOS damage response system. In the intact state, dimerization of the C-terminal domains ensures that when the repressor binds to DNA, its two N-terminal domains each contact DNA simultaneously. Cleavage releases the Cterminal domains from the N-terminal domains, though. As illustrated in FIGURE 2, this means that the N-terminal domains can no longer dimerize, which upsets the equilibrium between monomers and dimers. As a result, they do not have sufficient affinity for the lambda repressor to remain bound to DNA, which allows the lytic cycle to start. Also, two dimers usually cooperate to bind at an operator, and the cleavage destabilizes this interaction.

FIGURE 1. Repressor dimers bind to the operator. The affinity of the N-terminal domains for DNA is controlled by the dimerization of the C-terminal domains.
The balance between lysogeny and the lytic cycle depends on the concentration of repressor. Intact repressor is present in a lysogenic cell at a concentration sufficient to ensure that the operators are occupied. If the repressor is cleaved, however, this concentration is inadequate, because of the lower affinity of the separate N-terminal domain for the operator. A concentration of repressor that is too high would make it impossible to induce the lytic cycle in this way; a level that is too low, of course, would make it impossible to maintain lysogeny.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


