

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
r-Protein Synthesis Is Controlled by Autoregulation
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
5-6-2021
2107
r-Protein Synthesis Is Controlled by Autoregulation
KEY CONCEPT
- Translation of an r-protein operon can be controlled by a product of the operon that binds to a site on the polycistronic mRNA.
About 70 or so proteins constitute the apparatus for bacterial gene expression. The ribosomal proteins are the major component, together with the ancillary proteins involved in protein synthesis. The subunits of RNA polymerase and its accessory factors make up the remainder. The genes coding for ribosomal proteins, protein synthesis factors, and RNA polymerase subunits all are intermingled and organized into a small number of operons. Most of these proteins are represented only by single genes in E. coli.
Coordinate controls ensure that these proteins are synthesized in amounts appropriate for the growth conditions: When bacteria grow more rapidly, they devote a greater proportion of their efforts to the production of the apparatus for gene expression. An array of mechanisms is used to control the expression of the genes coding for this apparatus and to ensure that the proteins are synthesized at comparable levels that are related to the levels of the rRNAs.
The organization of six operons is shown in FIGURE 1. About half of the genes for ribosomal proteins (r-proteins) map to four operons that lie close together (named str, spc, S10, and α simply for the first one of the functions to have been identified in each case). The rif and L11 operons lie together at another location.
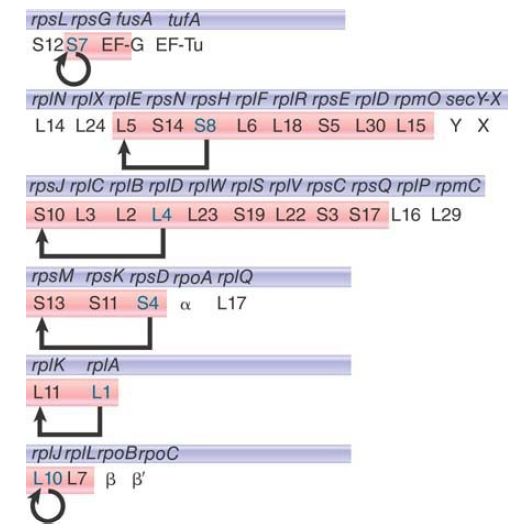
FIGURE 1.Genes for ribosomal proteins, protein synthesis factors, and RNA polymerase subunits are interspersed in a small number of operons that are autonomously regulated. The regulator is shaded in blue; the proteins that are regulated are shaded in pink.
Each operon codes for a variety of functions. The str operon has genes for small subunit ribosomal proteins, as well as for EF-Tu and EF-G. The spc and S10 operons have genes interspersed for both small and large ribosomal subunit proteins. The α operon has genes for proteins of both ribosomal subunits, as well as for the α subunit of RNA polymerase. The rif locus has genes for large subunit ribosomal proteins and for the β and β′ subunits of RNA polymerase.
All except one of the ribosomal proteins are needed in equimolar amounts, which must be coordinated with the level of rRNA. The dispersion of genes whose products must be equimolar, and their intermingling with genes whose products are needed in different amounts, pose some interesting problems for coordinate regulation.
A feature common to all of the operons described in Figure 1 is regulation of some of the genes by one of the products. In each case, the gene coding for the regulatory product is itself one of the targets for regulation. Autoregulation occurs whenever a protein (or RNA) regulates its own production. In the case of the r-protein operons, the regulatory protein inhibits expression of a contiguous set of genes within the operon, so this is an example of negative autoregulation.
In each case, accumulation of the protein inhibits further synthesis of itself and of some other gene products. The effect often is exercised at the level of translation of the polycistronic mRNA. Each of the regulators is a ribosomal protein that binds directly to rRNA. Its effect on translation is a result of its ability also to bind to its own mRNA. The sites on mRNA at which these proteins bind either overlap the sequence where translation is initiated or lie nearby and probably influence the accessibility of the initiation site by inducing conformational changes. For example, in the S10 operon, protein L4 acts at the very start of the mRNA to inhibit translation of S10 and the subsequent genes. The inhibition may result from a simple block to ribosome access, as illustrated in the Translation chapter, or it may prevent a subsequent stage of translation. In two cases (including S4 in the α operon), the regulatory protein stabilizes a particular secondary structure in the mRNA that prevents the initiation reaction from continuing after the 30S subunit has bound.
The use of r-proteins that bind rRNA to establish autogenous regulation immediately suggests that this provides a mechanism to link r-protein synthesis to rRNA synthesis. A generalized model is depicted in FIGURE 24.41. Suppose that the binding sites for the autogenous regulator r-proteins on rRNA are much stronger than those on the mRNAs. As long as any free rRNA is available, the newly synthesized r-proteins will associate with it to start ribosome assembly. No free r-protein will be available to bind to the mRNA, so its translation will continue. As soon as the synthesis of rRNA slows or stops, though, free r-proteins begin to accumulate. They are then available to bind their mRNAs and thus repress further translation. This circuit ensures that each r-protein operon responds in the same way to the level of rRNA: As soon as there is an excess of r-protein relative to rRNA, synthesis of the protein is repressed.
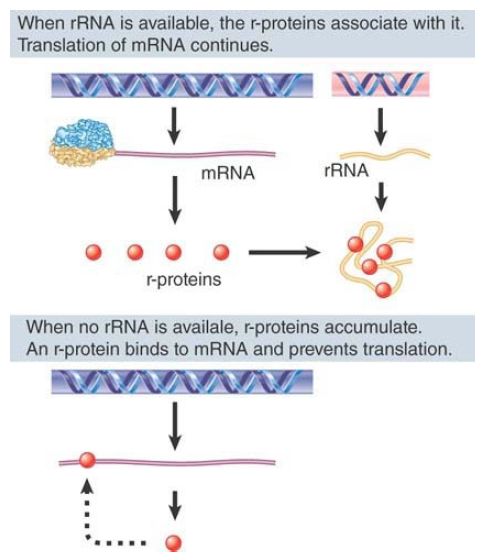
FIGURE 2.Translation of the r-protein operons is autogenously controlled and responds to the level of rRNA.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















