

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Protein Splicing Is Autocatalytic
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
22-5-2021
2473
Protein Splicing Is Autocatalytic
KEY CONCEPTS
- An intein has the ability to catalyze its own removal from a protein in such a way that the flanking exteins are connected.
- Protein splicing is catalyzed by the intein.
- Most inteins have two independent activities: protein splicing and a homing endonuclease.
Protein splicing has the same effect as RNA splicing: A sequence that is represented within the gene fails to be represented in the protein. The parts of the protein are named by analogy with RNA splicing: Exteins are the sequences that are represented in the mature protein, and inteins are the sequences that are removed. The mechanism of removing the intein is completely different from that of RNA splicing. FIGURE 1 shows that the gene is transcribed and translated into a protein precursor that contains the intein, and then the intein is excised from the protein. More than 500 examples of protein splicing have been identified, spread throughout all three domains. The typical gene whose product undergoes protein splicing has a single intein.
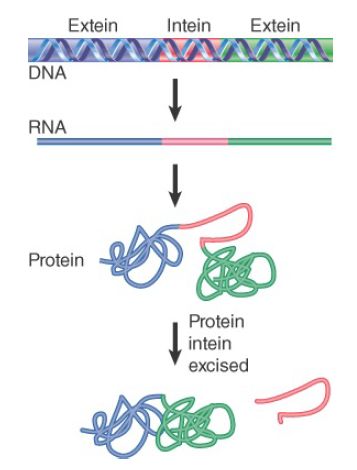
FIGURE 1. In protein splicing, the exteins are connected by removing the intein from the protein.
The first intein was discovered in an archaeal DNA polymerase gene in the form of an intervening sequence in the gene that does not conform to the rules for introns. It was then demonstrated that the purified protein can splice this sequence out of itself in an autocatalytic reaction. The reaction does not require input of energy and occurs through the series of bond rearrangements shown in FIGURE 2. The reaction is a function of the intein, although its efficiency can be influenced by the exteins.
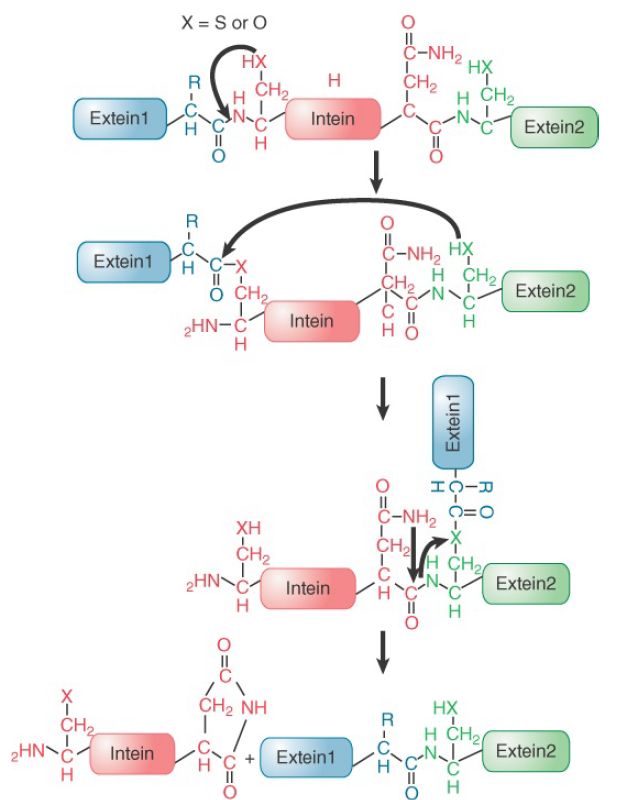
FIGURE 2. Bonds are rearranged through a series of transesterifications involving the –OH groups of serine or threonine or the –SH group of cysteine until the exteins are connected by a peptide bond and the intein is released with a circularized Cterminus.
The first reaction is an attack by an –OH or –SH side chain of the first amino acid in the intein on the peptide bond that connects it to the first extein. This transfers the extein from the amino-terminal group of the intein to an N–O or N–S acyl connection. This bond is then attacked by the –OH or –SH side chain of the first amino acid in the second extein. The result is to transfer extein1 to the side chain of the amino-terminal acid of extein2. Finally, the C-terminal asparagine of the intein cyclizes, and the terminal –NH of extein2 attacks the acyl bond to replace it with a conventional peptide bond. Each of these reactions can occur spontaneously at very low rates, but their occurrence in a coordinated manner that is rapid enough to achieve protein splicing requires catalysis by the intein. Inteins have characteristic features. They are found as in-frame insertions into coding sequences. They can be recognized as such because of the existence of homologous genes that lack the insertion. They have an N-terminal serine or cysteine (to provide the –OH or –SH side chain) and a C-terminal asparagine. A typical intein has a sequence of about 150 amino acids at the N-terminal end and about 50 amino acids at the C-terminal end that are involved in catalyzing the protein-splicing reaction. The sequence in the center of the intein can have other functions. Additionally, protein splicing can be performed in trans if the intein is split between two separate proteins. The two halves of these “split inteins” interact, allowing trans-splicing to form a single intact protein and a free intein. At least two split inteins have been identified in nature, and a number of other split inteins have been artificially engineered. Split inteins are of significant interest for protein engineers as they allow two separate peptides to be covalently fused in vivo.
An extraordinary feature of many inteins is that they have homing endonuclease activity. A homing endonuclease cleaves a target DNA to create a site into which the DNA sequence encoding the intein can be inserted . The protein-splicing and homing endonuclease activities of an intein are independent. The connection between these two activities in an intein is not well understood, but two types of model have been suggested. One is to suppose that there was originally some sort of connection between the activities, but that they have since become independent and some inteins have lost the homing endonuclease. The other is to suppose that inteins may have originated as proteinsplicing units, most of which (for unknown reasons) were subsequently invaded by homing endonucleases. This is consistent with the fact that homing endonucleases appear to have invaded other types of units as well, including, most notably, group I introns.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















