
Messenger RNAs Are Unstable Molecules
 المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
 المصدر:
LEWIN’S GENES XII
المصدر:
LEWIN’S GENES XII
 الجزء والصفحة:
الجزء والصفحة:
 18-5-2021
18-5-2021
 2540
2540
Messenger RNAs Are Unstable Molecules
KEY CONCEPTS
- mRNA instability is due to the action of ribonucleases.
- Ribonucleases differ in their substrate preference and mode of attack.
- mRNAs exhibit a wide range of half-lives.
- Differential mRNA stability is an important contributor to mRNA abundance, and therefore the spectrum of proteins made in a cell.
Messenger RNAs are relatively unstable molecules, unlike DNA, and, to a lesser extent, rRNAs and tRNAs. Although it is true that the phosphodiester bonds connecting ribonucleotides are somewhat weaker than those connecting deoxyribonucleotides due to the presence of the 2′–OH group on the ribose sugar, this is not the primary reason for the instability of mRNA. Rather, cells contain myriad RNA-degrading enzymes, called ribonucleases (RNases), some of which specifically target mRNA molecules.
Ribonucleases are enzymes that cleave the phosphodiester linkage connecting RNA ribonucleotides. They are diverse molecules because many different protein domains have evolved to have ribonuclease activity. The rare examples of known ribozymes (catalytic RNAs) include multiple ribonucleases, indicating the ancient origins of this important activity . Ribonucleases, often just called nucleases when the RNA nature of the substrate is obvious, have many roles in a cell, including participation in DNA replication, DNA repair, processing of new transcripts (including pre-mRNAs, tRNAs,rRNAs, snRNAs, and miRNAs), and the degradation of mRNA.
Ribonucleases are either endoribonucleases or exoribonucleases, as depicted in FIGURE 1 (and as discussed in the chapter titled Methods in Molecular Biology and Genetic Engineering). Endonucleases cleave an RNA molecule at an internal site and may have a requirement or preference for a certain structure or sequence. Exonucleases remove nucleotides from an RNA terminus and have a defined polarity of attack—either 5′ to 3′ or 3′ to 5′. Some exonucleases are processive, remaining engaged with the substrate while sequentially removing nucleotides, whereas others are distributive, catalyzing the removal of only one or a few nucleotides before dissociating from the substrate.
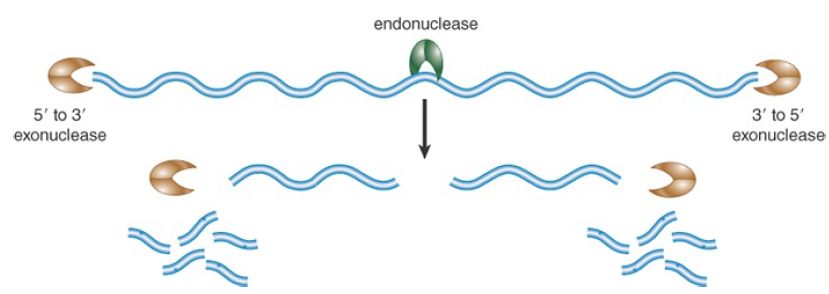
FIGURE 1. Types of ribonucleases. Exonucleases are unidirectional. They can digest RNA either from the 5′ end or from the 3′ end, liberating individual ribonucleotides. Endonucleases cleave RNA at internal phosphosphodiester linkages. An endonuclease usually targets specific sequences and/or secondary structures.
Most mRNAs decay stochastically (like the decay of radioactive isotopes), and as a result mRNA stability is usually expressed as a half-life (t½ ). The term mRNA decay is often used interchangeably with mRNA degradation. mRNA-specific stability information is encoded in cis-sequences and is therefore characteristic of each mRNA. Different mRNAs can exhibit remarkably different stabilities, varying by 100-fold or more. In E. coli the typical mRNA half-life is about 3 minutes, but half-lives of individual mRNAs may be as short as 20 seconds or as long as 90 minutes. In budding yeast, mRNA half-lives range from 3 to 100 minutes, whereas in metazoans half-lives range from minutes to hours, and in rare cases, even days. Abnormal mRNAs can be targeted for very rapid destruction . Half-life values are generally determined by some version of the method illustrated in FIGURE 2.
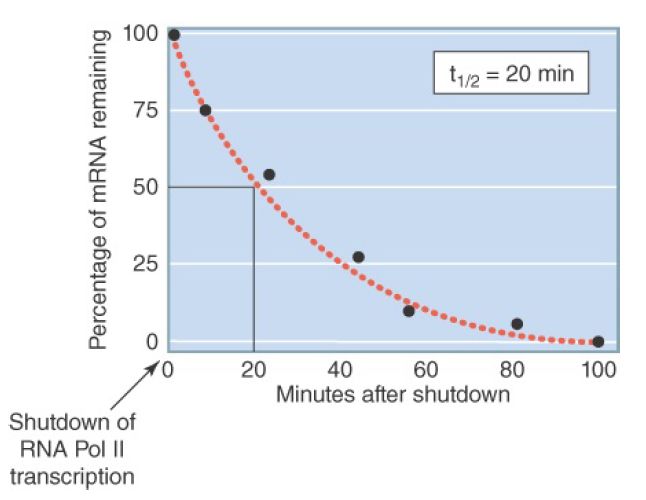
FIGURE 2. Method for determining mRNA half-lives. RNA polymerase II transcription is shut down, either by a drug or a temperature shift in strains with a temperature-sensitive mutation in a Pol II gene. The levels of specific mRNAs are determined by northern blot or RT-PCR at various times following shutdown. RNA degradation, once initiated, is usually so rapid that intermediates in the process are not detectible. The half-life is the time required for the mRNA to fall to one-half of its initial value.
The abundance of specific mRNAs in a cell is a consequence of their combined rates of synthesis (transcription and processing) and degradation. mRNA levels reach a steady state when these parameters remain constant. The spectrum of proteins synthesized by a cell is largely a reflection of the abundance of their mRNA templates (although differences in translational efficiency play a role). The importance of mRNA decay is highlighted by large-scale studies that have examined the relative contributions of decay rate and transcription rate to differential mRNA abundance. Decay rate predominates. The great advantage of unstable mRNAs is the ability to rapidly change the output of translation through changes in mRNA synthesis. Clearly this advantage is important enough to compensate for the seeming wastefulness of making and destroying mRNAs so quickly. Abnormal control of mRNA stability has been implicated in disease states, including cancer, chronic inflammatory responses, and coronary disease.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


