
DNA Structure Varies on the Nucleosomal Surface
 المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
 المصدر:
LEWIN’S GENES XII
المصدر:
LEWIN’S GENES XII
 الجزء والصفحة:
الجزء والصفحة:
 25-3-2021
25-3-2021
 2372
2372
DNA Structure Varies on the Nucleosomal Surface
KEY CONCEPTS
-DNA is wrapped 1.67 times around the histone octamer.
-DNA on the nucleosome shows regions of smooth curvature and regions of abrupt kinks.
-The structure of the DNA is altered so that it has an increased number of bp/turn in the middle, but a decreased number at the ends.
-Approximately 0.6 negative turns of DNA are absorbed by the change in bp/turn from 10.5 in solution to an average of 10.2 on the nucleosomal surface, which explains the linking-number paradox.
So far, we have focused on the protein components of the nucleosome. The DNA wrapped around these proteins is in an unusual conformation. The exposure of DNA on the surface of the nucleosome explains why it is accessible to cleavage by certain nucleases. The reaction with nucleases that attack single strands has been especially informative. The enzymes DNase I and DNase II make single-strand nicks in DNA; they cleave a bond in one strand, but the other strand remains intact. No effect is visible in linear double-stranded DNA, but when this DNA is denatured, shorter fragments are released instead of full-length single strands. If the DNA has been labeled at its ends, the end fragments can be identified by detection of the label, as summarized in FIGURE 1.
When DNA is free in solution, it is nicked (relatively) at random. The DNA on nucleosomes can also be nicked by the enzymes, but only at regular intervals. When the points of cutting are determined by using end-labeled DNA and the DNA is denatured and electrophoresed, a ladder of the sort displayed in FIGURE 2 isobtained.
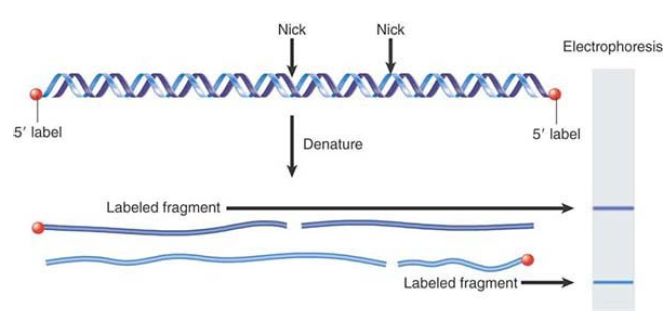
FIGURE 1 Nicks in double-stranded DNA are revealed by fragments when the DNA is denatured to give single strands. For example, if the DNA is labeled at the 5′ ends, only the 5′ fragments are visible by autoradiography. The size of the fragment identifies the distance of the nick from the labeled end.

FIGURE 2 Sites for nicking lie at regular intervals along core DNA, as seen in a DNase I digest of nuclei.
Photo courtesy of Leonard C. Lutter, Molecular Biology Research Program, Henry Ford Hospital.
The interval between successive steps on the ladder is 10–11 bases. The ladder extends for the full distance of core DNA. The cleavage sites are numbered as S1 through S12 (where S1 is 10–11 bases from the labeled 5′ end, S2 is about 20 bases from it, and so on). The enzymes DNase I and DNase II generate essentially the same ladder, and the same pattern is obtained by cleaving with a hydroxyl radical, which argues that the pattern reflects the structure of the DNA itself rather than any sequence preference. The sensitivity of nucleosomal DNA to nucleases is analogous to a footprinting experiment. Thus, we can assign the lack of reaction at particular target sites to the structure of the nucleosome, in which certain positions on DNA are rendered inaccessible.
There are two strands of DNA in the core particle, so in an endlabeling experiment both of the 5′ (or 3′) ends are labeled, one on each strand. Thus, the cutting pattern includes fragments derived from both strands. This is visible in Figure 1, in which each labeled fragment is derived from a different strand. The corollary is that, in an experiment, each labeled band might actually represent two fragments that are generated by cutting the same distance from either of the labeled ends.
How, then, should we interpret discrete preferences at particular sites? One view is that the path of DNA on the particle is symmetrical . If, for example, no 80-base fragment is generated by DNase I, this must mean that the position at 80 bases from the 5′ end of either strand is not susceptible to the enzyme.
When DNA is immobilized on a flat surface, sites are cut with a regular separation. FIGURE 3 shows that this reflects the recurrence of the exposed site with the helical periodicity of B-formDNA. The cu tting periodicity (the spacing between cleavage points) coincides with—indeed, is a reflection of—the structural periodicity (the number of base pairs per turn of the double helix). Thus, the distance between the sites corresponds to the number of base pairs per turn. Measurements of this type yield the average value for double-helical B-type DNA of 10.5 bp/turn.

FIGURE 3 The most exposed positions on DNA recur with a periodicity that reflects the structure of the double helix. (For clarity, sites are shown for only one strand.)
A similar analysis of DNA on the surface of the nucleosome reveals striking variations in the structural periodicity at different points. At the ends of the DNA, the average distance between pairs of DNase I digestion sites is about 10.0 bases each, significantly less than the usual 10.5 bp/turn. In the center of the particle, the separation between cleavage sites averages 10.7 bases. This variation in cutting periodicity along the core DNA means that there is variation in the structural periodicity of core DNA. The DNA has more bp/turn than its solution value in the middle, but has fewer bp/turn at the ends. The average periodicity over the entire nucleosome is only 10.17 bp/turn, which is significantly less than the 10.5 bp/turn of DNA in solution.
The crystal structure of the core particle shows that DNA is wound into a solenoidal (spring-shaped) supercoil, with 1.67 turns wound around the histone octamer. The pitch of the superhelix varies and has a discontinuity in the middle. Regions of high curvature are arranged symmetrically and are the sites least sensitive to DNase I.
The high-resolution structure of the nucleosome core shows in detail how the structure of DNA is distorted. Most of the supercoiling occurs in the central 129 bp, which are coiled into 1.59 left-handed superhelical turns with a diameter of 80 Å (only four times the diameter of the DNA duplex itself). The terminal sequences on either end make only a very small contribution to the overall curvature.
The central 129 bp are in the form of B-DNA, but with a substantial curvature that is needed to form the superhelix. The major groove is smoothly bent, but the minor groove has abrupt kinks, as shown in FIGURE 4. These conformational changes might explain why the central part of nucleosomal DNA is not usually a target for binding by regulatory proteins, which typically bind to the terminal parts of the core DNA or to the linker sequences.
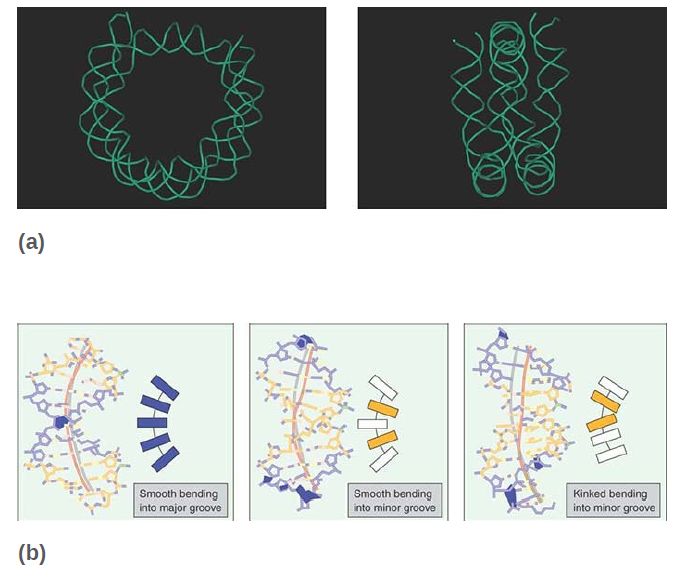
FIGURE 4 DNA structure in nucleosomal DNA. (a) The trace of the DNA backbone in the nucleosome is shown in the absence of protein for clarity. (b) Regions of curvature in nucleosomal DNA. Actual structures (left) and schematic representations (right) show uniformity of curvature along the major groove (blue) and both smooth and kinked bending into the minor groove (orange). Also indicated are the DNA axes for the experimental (pink) and ideal (gray) superhelices.
(a) Data from: Muthurajan, U. M., et al. 2004. “Structures from Protein Data Bank: 1P34.” EMBO J 23:260–271.
(b) Data from: Richmond, T. J., and Davey, C. A. 2003. Nature 423:145–150.
Some insights into the structure of nucleosomal DNA emerge when we compare predictions for supercoiling in the path that DNA follows with actual measurements of supercoiling of nucleosomal DNA. Circular “minichromosomes” that are fully assembled into nucleosomes can be isolated from eukaryotic cells. Researchers can measure the degree of supercoiling on the individual nucleosomes of the minichromosome as illustrated in FIGURE 5. First, the free supercoils of the minichromosome itself are relaxed, so that the nucleosomes form a circular string with an unconstrained superhelical density of 0. Next, the histone octamers are extracted. This releases the DNA to follow a free path. Every negative supercoil that was present but constrained in thenucleosomes will appe ar in the deproteinized DNA as −1 turn. Now the total number of supercoils in the DNA is measured.
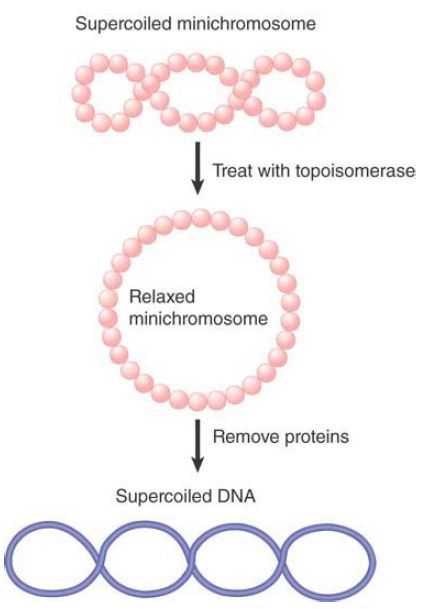
FIGURE 5 The supercoils of the SV40 minichromosome can be relaxed to generate a circular structure, whose loss of histones then generates supercoils in the free DNA.
The observed value is close to the number of nucleosomes. Thus, the DNA follows a path on the nucleosomal surface that generates about one negative supercoiled turn when the restraining protein is removed. The path that DNA follows on the nucleosome, however, corresponds to −1.67 superhelical turns. This discrepancy is sometimes called the linking number paradox.
The discrepancy is explained by the difference between the 10.17a verage bp/turn of nucleosomal DNA and the 10.5 bp/turn of free DNA. In a nucleosome of 200 bp, there are 200/10.17 = 19.67 turns. When DNA is released from the nucleosome, it now has 200/10.5 = 19.0 turns. The path of the less tightly wound DNA on the nucleosome absorbs −0.67 turns, which explains the discrepancy between the physical path of −1.67 and the measurement of −1.0 superhelical turns. In effect, some of the torsional strain in nucleosomal DNA goes into increasing the number of bp/turn; only the rest is left to be measured as a supercoil.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


