

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Many Hotspots Result from Modified Bases
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
27-2-2021
3313
Many Hotspots Result from Modified Bases
KEY CONCEPTS
-A common cause of hotspots is the modified base 5- methylcytosine, which is spontaneously deaminated to thymine.
-A hotspot can result from imprecise replication of a short, tandemly repeated sequence.
A major cause of spontaneous mutation is the presence of an unusual base in the DNA. In addition to the four standard bases of DNA, modified bases are sometimes found. The name reflects their origin; they are produced by chemical modification of one of the four standard bases. The most common modified base is 5-methylcytosine, which is generated when a methyltransferase enzyme adds a methyl group to cytosine residues at specific sites in the DNA. Sites containing 5-methylcytosine are hotspots for spontaneous point mutation in E. coli. In each case, the mutation is a G-C to A-T transition. The hotspots are not found in mutant strains of E. coli that cannot methylate cytosine.
The reason for the existence of these hotspots is that cytosine bases suffer a higher frequency of spontaneous deamination. In this reaction, the amino group is replaced by a keto group. Recall that deamination of cytosine generates uracil . FIGURE 1. compares this reaction with the deamination of 5-methylcytosine where deamination generates thymine. The effect is to generate the mismatched base pairs G-U and G-T, respectively.
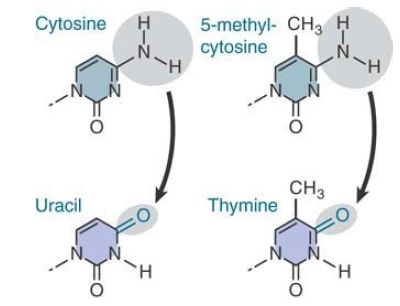
FIGURE 1. Deamination of cytosine produces uracil, whereas deamination of 5-methylcytosine produces thymine.
All organisms have repair systems that correct mismatched base pairs by removing and replacing one of the bases . The operation of these systems determines whether mismatched pairs such as G-U and G-T persist into the next round of DNA replication and thereby result in mutations.
FIGURE 2. shows that the consequences of deamination are different for 5-methylcytosine and cytosine. Deaminating the (rare) 5-methylcytosine causes a mutation, whereas deaminating cytosine does not have this effect. This happens because the DNA repair systems are much more effective in accurately repairing G-U than G-T base pairs.
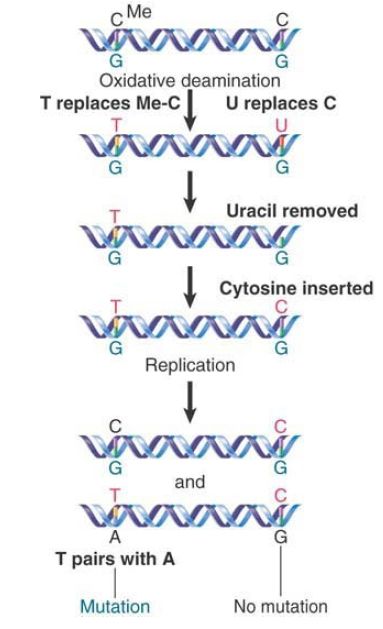
FIGURE 2. The deamination of 5-methylcytosine produces thymine (by C-G to T-A transitions), whereas the deamination of cytosine produces uracil (which usually is removed and then replaced by cytosine).
E. coli contain an enzyme, uracil-DNA-glycosidase, that removes uracil residues from DNA. This action leaves an unpaired G residue, and a repair system then inserts a complementary C base.
The net result of these reactions is to restore the original sequence of the DNA. Thus, this system protects DNA against the consequences of spontaneous deamination of cytosine. (This
system is not, however, efficient enough to prevent the effects of the increased deamination caused by nitrous acid.)
Note that the deamination of 5-methylcytosine creates thymine and results in a mismatched base pair, G-T. If the mismatch is not corrected before the next replication cycle, a mutation results. The bases in the mispaired G-T first separate and then pair with the correct complements to produce the wild-type G-C in one daughter DNA and the mutant A-T in the other.
Deamination of 5-methylcytosine is the most common cause of mismatched G-T pairs in DNA. Repair systems that act on G-T mismatches have a bias toward replacing the T with a C (rather
than the alternative of replacing the G with an A), which helps to reduce the rate of mutation . However, these systems are not as effective as those that remove U from G-U mismatches. As a result, deamination of 5-methylcytosine leads to mutation much more often than does deamination of cytosine.
Additionally, 5-methylcytosine creates hotspots in eukaryotic DNA. It is common in CpG dinucleotide repeats that are concentrated in regions called CpG islands (see the chapter titled Epigenetics I Effects Are Inherited). Although 5-methylcytosine accounts for about 1% of the bases in human DNA, sites containing the modified base account for about 30% of all point mutations.
The importance of repair systems in reducing the rate of mutation is emphasized by the effects of eliminating the mouse enzyme MBD4, a glycosylase that can remove T (or U) from mismatches with G. The result is to increase the mutation rate at CpG sites by a factor of 3. The reason the effect is not greater is that MBD4 is only one of several systems that act on G-T mismatches; most likely the elimination of all the systems would increase the mutation rate much more.
The operation of these systems casts an interesting light on the use of T in DNA as compared to U in RNA. It might relate to the need for stability of DNA sequences; the use of T means that any deaminations of C are immediately recognized because they generate a base (U) that is not usually present in the DNA. This greatly increases the efficiency with which repair systems can function (compared with the situation when they have to recognize G-T mismatches, which can also be produced by situations in which removing the T would not be the appropriate correction). In addition, the phosphodiester bond of the backbone is more easily broken when the base is U.
Another type of hotspot, though not often found in coding regions, is the “slippery sequence”—a homopolymer run, or region where a very short sequence (one or a few nucleotides) is repeated many times in tandem. During replication, a DNA polymerase can skip
one repeat or replicate the same repeat twice, leading to a decrease or increase in repeat number.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















