
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء والفلسفة

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
The Graphite Crystal
المؤلف:
E. R. Huggins
المصدر:
Physics 2000
الجزء والصفحة:
914
30-12-2020
2360
The Graphite Crystal
Graphite makes an ideal substance to study by electron scattering because graphite crystals come in thin sheets. A graphite crystal consists of a series of planes of carbon atoms. Within one plane the atoms have the hexagonal structure shown in Figure (1), reminiscent of the tiles often seen on bathroom floors. The spacing between neighboring atoms in each hexagon is 1.42 Ao as indicated at the bottom of Figure (1).
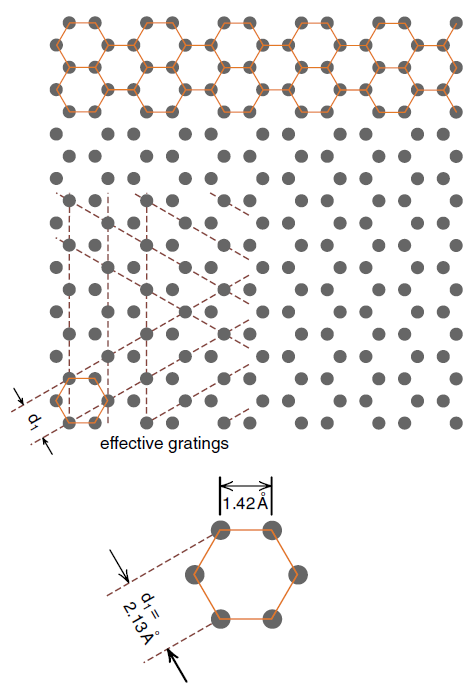
Figure 1: The hexagonal array of atoms in one layer of a graphite crystal. Lines of atoms in this crystal act as crossed diffraction gratings.
The atoms within a plane are very tightly bound together. The hexagonal array forms a very strong framework. The planes themselves are stacked on top of each other at the considerable distance of 3.63 Ao as indicated in Figure (2). The forces between these planes are weak, allowing the planes to easily slide over each other. The result is that graphite is a slippery substance, making an excellent dry lubricant. In contrast, the strength within a plane makes graphite an excellent strengthening agent for epoxy. The resulting carbon filament epoxies, used for constructing racing boat hulls, light airplanes and stayless sailboat masts, is one of the strongest plastics available.

Figure 2: Edge view of the graphite crystal, showing the planes of atoms. The planes can easily slide over each other, making the substance slippery.
 الاكثر قراءة في مواضيع عامة في الفيزياء الصلبة
الاكثر قراءة في مواضيع عامة في الفيزياء الصلبة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















