

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Friedel–Crafts reaction
المؤلف:
University of Missouri System
المصدر:
Organic Chemistry ii
الجزء والصفحة:
.................
27-9-2020
3022
Friedel–Crafts reaction
Friedel–Crafts alkylation
Friedel–Crafts alkylation involves the alkylation of an aromatic ring with an alkyl halide using a strong Lewis acid catalyst.[6] With anhydrous ferric chloride as a catalyst, the alkyl group attaches at the former site of the chloride ion. The general mechanism is shown below.

This reaction suffers from the disadvantage that the product is more nucleophilic than the reactant. Consequently, overalkylation occurs. Furthermore, the reaction is only very useful for tertiary alkylating agents, some secondary alkylating agents, or ones that yield stabilized carbocations (e.g., benzylic ones). In the case of primary alkyl halides, the incipient carbocation (R(+)—X—Al(-)Cl3) will undergo a carbocation rearrangement reaction.
Steric hindrance can be exploited to limit the number of alkylations, as in the t-butylation of 1,4-dimethoxybenzene.

Alkylations are not limited to alkyl halides: Friedel–Crafts reactions are possible with any carbocationic intermediate such as those derived from alkenes and a protic acid, Lewis acid, enones, and epoxides. An example is the synthesis of neophyl chloride from benzene and methallyl chloride:
H2C=C(CH3)CH2Cl + C6H6 → C6H5C(CH3)2CH2Cl
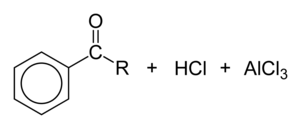
Friedel–Crafts acylation
Friedel–Crafts acylation involves the acylation of aromatic rings. Typical acylating agents are acyl chlorides. Typical Lewis acid catalysts are acids and aluminium trichloride. Friedel–Crafts acylation is also possible with acid anhydrides.[11] Reaction conditions are similar to the Friedel–Crafts alkylation. This reaction has several advantages over the alkylation reaction. Due to the electron-withdrawing effect of the carbonyl group, the ketone product is always less reactive than the original molecule, so multiple acylations do not occur. Also, there are no carbocation rearrangements, as the acylium ion is stabilized by a resonance structure in which the positive charge is on the oxygen.

The viability of the Friedel–Crafts acylation depends on the stability of the acyl chloride reagent. Formyl chloride, for example, is too unstable to be isolated. Thus, synthesis of benzaldehyde through the Friedel–Crafts pathway requires that formyl chloride be synthesized in situ. This is accomplished by the Gattermann-Koch reaction, accomplished by treating benzene with carbon monoxide and hydrogen chloride under high pressure, catalyzed by a mixture of aluminium chloride and cuprous chloride.
Reaction mechanism
The reaction proceeds through generation of an acylium center:

The reaction is completed by deprotonation of the arenium ion by AlCl4−, regenerating the AlCl3 catalyst:
If desired, the resulting ketone can be subsequently reduced to the corresponding alkane substituent by either Wolff–Kishner reduction or Clemmensen reduction. The net result is the same as the Friedel–Crafts alkylation except that rearrangement is not possible.
Limitations on Electrophilic Substitution Reactions with Substituted Benzenes
Over reaction of Aniline and Phenol
The strongest activating and ortho/para-directing substituents are the amino (-NH2) and hydroxyl (-OH) groups.
By acetylating the heteroatom substituent on phenol and aniline, its activating influence can be substantially attenuated. For example, acetylation of aniline gives acetanilide (first step in the following equation), which undergoes nitration at low temperature, yielding the para-nitro product in high yield. The modifying acetyl group can then be removed by acid-catalyzed hydrolysis (last step), to yield para-nitroaniline. Although the activating influence of the amino group has been reduced by this procedure, the acetyl derivative remains an ortho/para-directing and activating substituent.
| C6H5–NH2 + (CH3CO)2O | pyridine (a base) |
C6H5–NHCOCH3 | HNO3 , 5 ºC |
p-O2N–C6H4–NHCOCH3 | H3O(+) & heat |
p-O2N–C6H4–NH |


Some limitations of Friedel-Crafts Alkylation
There are possibilities of carbocation rearrangements when you are trying to add a carbon chain greater than two carbons. The rearrangements occur due to hydride shifts and methyl shifts. For example, the product of a Friedel-Crafts Alkylation will show an iso rearrangement when adding a three carbon chain as a substituent. One way to resolve these problems is through Friedel-Crafts Acylation.
Also, the reaction will only work if the ring you are adding a substituent to is not deactivated. Friedel-Crafts fails when used with compounds such as nitrobenzene and other strong deactivating systems.
Friedel-Crafts reactions cannot be performed easily when the aromatic ring contains a NH2, NHR, or NR2 substituent. The lone pair electrons on the amines react with the Lewis acid AlCl3. This places a positive charge next to the benzene ring, which is so strongly activating that the Friedel-Crafts reaction cannot occur.
Lastly, Friedel-Crafts alkylation can undergo polyalkylation. The reaction adds an electron donating alkyl group, which activates the benzene ring to further alkylation.
This problem does not occur during Friedel-Crafts Acylation because an acyl group is deactivating. The prevents further acylations.
 الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















