

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Features of the Periodic Table
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
25-7-2020
1885
Features of the Periodic Table
Elements that have similar chemical properties are grouped in columns called groups (or families). As well as being numbered, some of these groups have names—for example, alkali metals (the first column of elements), alkaline earth metals (the second column of elements), halogens (the next-to-last column of elements), and noble gases (the last column of elements).
The word halogen comes from the Greek for “salt maker” because these elements combine with other elements to form a group of compounds called salts.
After smoking, radon is thought to be the second-biggest preventable cause of lung cancer in the United States. The American Cancer Society estimates that 10% of all lung cancers are related to radon exposure. There is uncertainty regarding what levels of exposure cause cancer, as well as what the exact causal agent might be (either radon or one of its breakdown products, many of which are also radioactive and, unlike radon, not gases). The US Environmental Protection Agency recommends testing every floor below the third floor for radon levels to guard against long-term health effects.
Each row of elements on the periodic table is called a period. Periods have different lengths; the first period has only 2 elements (hydrogen and helium), while the second and third periods have 8 elements each. The fourth and fifth periods have 18 elements each, and later periods are so long that a segment from each is removed and placed beneath the main body of the table.
Certain elemental properties become apparent in a survey of the periodic table as a whole. Every element can be classified as either a metal, a nonmetal, or a metalloid (or semi metal), as shown in Figure 1 . A metal is a substance that is shiny, typically (but not always) silvery in color, and an excellent conductor of electricity and heat. Metals are also malleable (they can be beaten into thin sheets) and ductile (they can be drawn into thin wires). A nonmetal is typically dull and a poor conductor of electricity and heat. Solid nonmetals are also very brittle. As shown in Figure 1 , metals occupy the left three-fourths of the periodic table, while nonmetals (except for hydrogen) are clustered in the upper right-hand corner of the periodic table. The elements with properties intermediate between those of metals and nonmetals are called metalloids (or semi-metals). Elements adjacent to the bold line in the right-hand portion of the periodic table have semimetal properties.
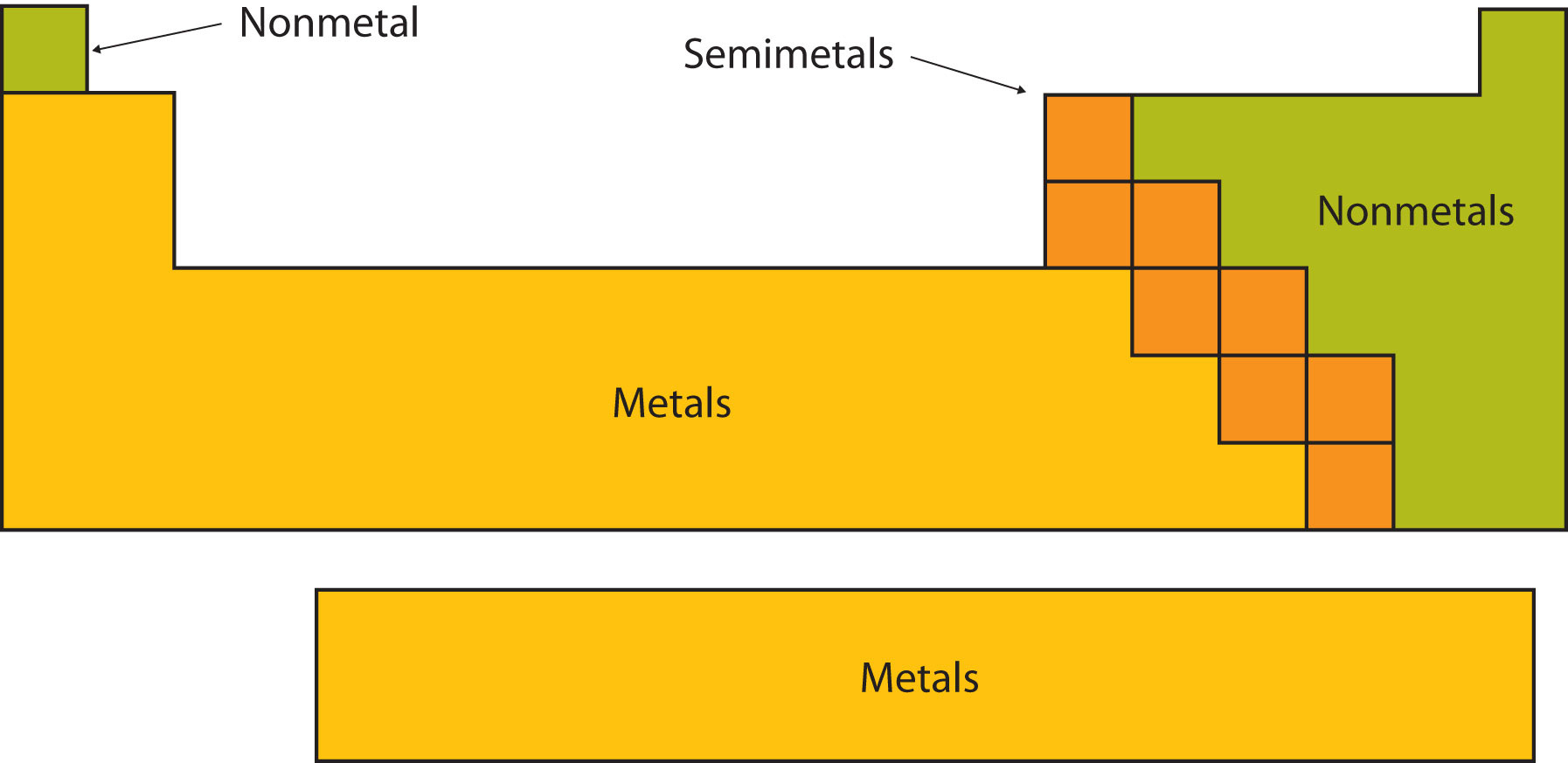
Figure 1 : Types of Elements. Elements are either metals, nonmetals, or metalloids (or semi metals). Each group is located in a different part of the periodic table. (CC BY-NC-SA; Anonymous by request)
Example .1: Based on its position in the periodic table, classify each element below as metal, a nonmetal, or a metalloid.
- Se
- Mg
- Ge
SOLUTION
- selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so it should be a nonmetal.
- Magnesium lies to the left of the diagonal line marking the boundary between metals and nonmetals, so it should be a metal.
- Germanium lies within the diagonal line marking the boundary between metals and nonmetals, so it should be a metalloid.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















