
Chemical Formula from Empirical Formula
 المؤلف:
LibreTexts Project
المؤلف:
LibreTexts Project
 المصدر:
................
المصدر:
................
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 5-7-2020
5-7-2020
 1564
1564
Chemical Formula from Empirical Formula
The chemical formula for a compound obtained by composition analysis is always the empirical formula. We can obtain the chemical formula from the empirical formula if we know the molecular weight of the compound. The chemical formula will always be some integer multiple of the empirical formula (i.e. integer multiples of the subscripts of the empirical formula). The general flow for this approach is shown in Figure 1 and demonstrated in Example 3.9.2 .
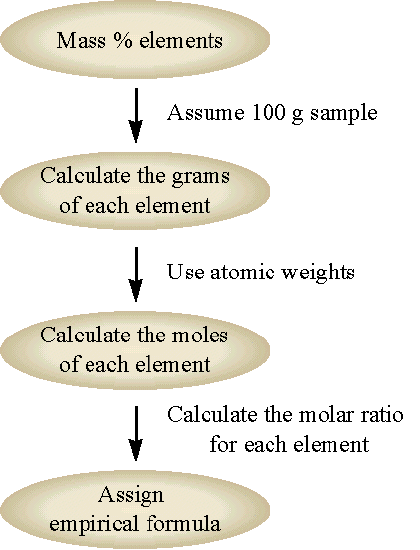
Figure 1 : The general flow chart for solving empirical formulas from known mass percentages.
Example 1: Ascorbic Acid
Vitamin C (ascorbic acid) contains 40.92 % C, 4.58 % H, and 54.50 % O, by mass. The experimentally determined molecular mass is 176 amu. What is the empirical and chemical formula for ascorbic acid?
Solution
Consider an arbitrary amount of 100 grams of ascorbic acid, so we would have:
- 40.92 grams C
- 4.58 grams H
- 54.50 grams O
This would give us how many moles of each element?

Determine the simplest whole number ratio by dividing by the smallest molar amount (3.406 moles in this case - see oxygen):

The relative molar amounts of carbon and oxygen appear to be equal, but the relative molar amount of hydrogen is higher. Since we cannot have "fractional" atoms in a compound, we need to normalize the relative amount of hydrogen to be equal to an integer. 1.333 would appear to be 1 and 1/3, so if we multiply the relative amounts of each atom by '3', we should be able to get integer values for each atom.
C = (1.0) * 3 = 3
H = (1.333) * 3 = 4
O = (1.0) * 3 = 3 or C3H4O3
This is our empirical formula for ascorbic acid. What about the chemical formula? We are told that the experimentally determined molecular mass is 176 amu. What is the molecular mass of our empirical formula?
(3*12.011) + (4*1.008) + (3*15.999) = 88.062 amu
The molecular mass from our empirical formula is significantly lower than the experimentally determined value. What is the ratio between the two values?
(176 amu/88.062 amu) = 2.0
Thus, it would appear that our empirical formula is essentially one half the mass of the actual molecular mass. If we multiplied our empirical formula by '2', then the molecular mass would be correct. Thus, the actual chemical formula is:
2* C3H4O3 = C6H8O6
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


