

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Coordination Compounds
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
3-6-2020
2329
Coordination Compounds
The hemoglobin in your blood, the chlorophyll in green plants, vitamin B12, and the catalyst used in the manufacture of polyethylene all contain coordination compounds. Ions of the metals, especially the transition metals, are likely to form complexes. Many of these compounds are highly colored (Figure 1.1 ). In the remainder of this chapter, we will consider the structure and bonding of these remarkable compounds.

Figure 1.1 : Metal ions that contain partially filled d subshell usually form colored complex ions; ions with empty d subshell (d0) or with filled d subshells (d10) usually form colorless complexes. This figure shows, from left to right, solutions containing [M(H2O)6]n+ ions with M = Sc3+(d0), Cr3+(d3), Co2+(d7), Ni2+(d8), Cu2+(d9), and Zn2+(d10). (credit: Sahar Atwa)
Remember that in most main group element compounds, the valence electrons of the isolated atoms combine to form chemical bonds that satisfy the octet rule. For instance, the four valence electrons of carbon overlap with electrons from four hydrogen atoms to form CH4. The one valence electron leaves sodium and adds to the seven valence electrons of chlorine to form the ionic formula unit NaCl (Figure 1.2 ). Transition metals do not normally bond in this fashion. They primarily form coordinate covalent bonds, a form of the Lewis acid-base interaction in which both of the electrons in the bond are contributed by a donor (Lewis base) to an electron acceptor (Lewis acid). The Lewis acid in coordination complexes, often called a central metal ion (or atom), is often a transition metal or inner transition metal, although main group elements can also form coordination compounds. The Lewis base donors, called ligands, can be a wide variety of chemicals—atoms, molecules, or ions. The only requirement is that they have one or more electron pairs, which can be donated to the central metal. Most often, this involves a donor atom with a lone pair of electrons that can form a coordinate bond to the metal.
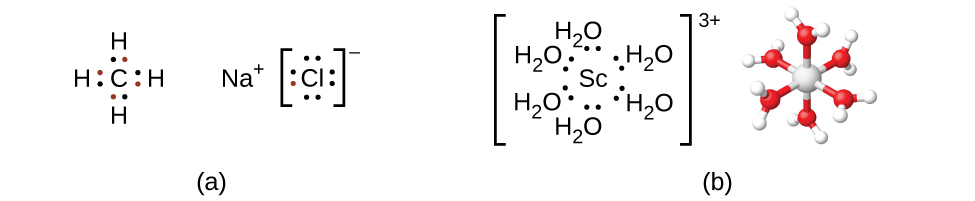
Figure 1.2 : (a) Covalent bonds involve the sharing of electrons, and ionic bonds involve the transferring of electrons associated with each bonding atom, as indicated by the colored electrons. (b) However, coordinate covalent bonds involve electrons from a Lewis base being donated to a metal center. The lone pairs from six water molecules form bonds to the scandium ion to form an octahedral complex. (Only the donated pairs are shown.)
The coordination sphere consists of the central metal ion or atom plus its attached ligands. Brackets in a formula enclose the coordination sphere; species outside the brackets are not part of the coordination sphere. The coordination number of the central metal ion or atom is the number of donor atoms bonded to it. The coordination number for the silver ion in [Ag(NH3)2]+ is two (Figure 1.3
). For the copper(II) ion in [CuCl4]2−, the coordination number is four, whereas for the cobalt(II) ion in [Co(H2O)6]2+ the coordination number is six. Each of these ligands is monodentate, from the Greek for “one toothed,” meaning that they connect with the central metal through only one atom. In this case, the number of ligands and the coordination number are equal.
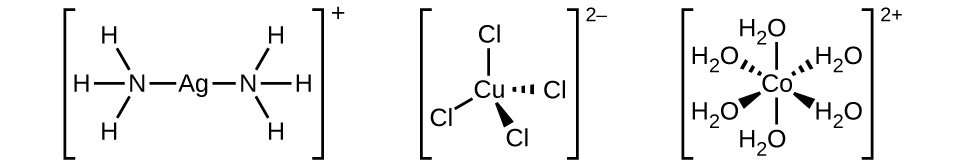
Figure 1.3 : The complexes (a) [Ag(NH3)2]+, (b) [Cu(Cl)4]2−, and (c) [Co(H2O)6]2+ have coordination numbers of two, four, and six, respectively. The geometries of these complexes are the same as we have seen with VSEPR theory for main group elements: linear, tetrahedral, and octahedral.
Many other ligands coordinate to the metal in more complex fashions. Bidentate ligands are those in which two atoms coordinate to the metal center. For example, ethylenediamine (en, H2NCH2CH2NH2) contains two nitrogen atoms, each of which has a lone pair and can serve as a Lewis base (Figure 1.4 ). Both of the atoms can coordinate to a single metal center. In the complex [Co(en)3]3+, there are three bidentate en ligands, and the coordination number of the cobalt(III) ion is six. The most common coordination numbers are two, four, and six, but examples of all coordination numbers from 1 to 15 are known.
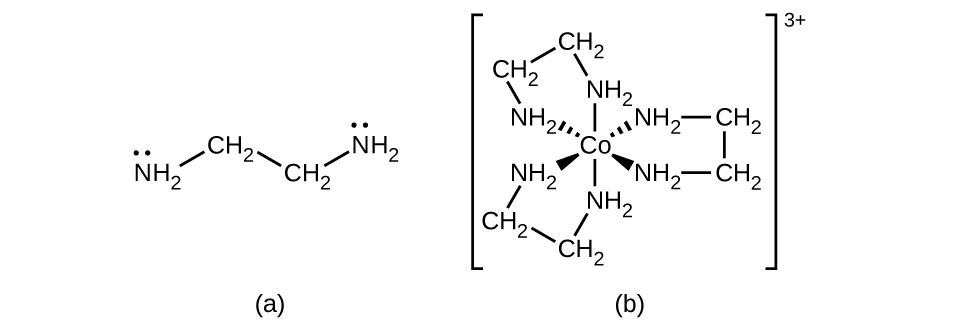
Figure 1.4 : (a) The ethylenediamine (en) ligand contains two atoms with lone pairs that can coordinate to the metal center. (b) The cobalt(III) complex [Co(en)3]3+ contains three of these ligands, each forming two bonds to the cobalt ion.
Any ligand that bonds to a central metal ion by more than one donor atom is a polydentate ligand (or “many teeth”) because it can bite into the metal center with more than one bond. The term chelate (pronounced “KEY-late”) from the Greek for “claw” is also used to describe this type of interaction. Many polydentate ligands are chelating ligands, and a complex consisting of one or more of these ligands and a central metal is a chelate. A chelating ligand is also known as a chelating agent. A chelating ligand holds the metal ion rather like a crab’s claw would hold a marble. Figure 1.4 showed one example of a chelate and the heme complex in hemoglobin is another important example (Figure 1.5). It contains a polydentate ligand with four donor atoms that coordinate to iron.
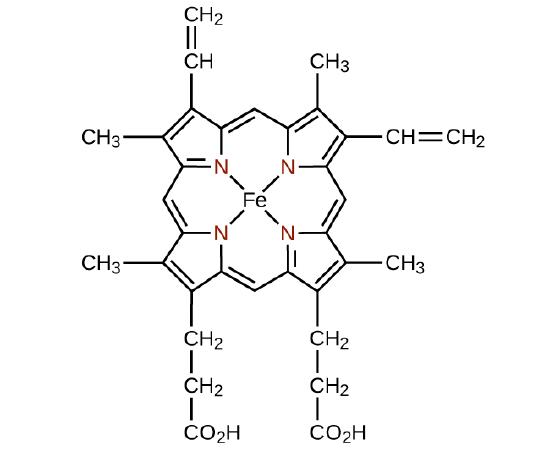
Figure 1.5 : The single ligand heme contains four nitrogen atoms that coordinate to iron in hemoglobin to form a chelate.
Polydentate ligands are sometimes identified with prefixes that indicate the number of donor atoms in the ligand. As we have seen, ligands with one donor atom, such as NH3, Cl−, and H2O, are monodentate ligands. Ligands with two donor groups are bidentate ligands. Ethylenediamine, H2NCH2CH2NH2, and the anion of the acid glycine, NH2CH2CO−2 (Figure 1.6) are examples of bidentate ligands. Tridentate ligands, tetradentate ligands, pentadentate ligands, and hexadentate ligands contain three, four, five, and six donor atoms, respectively. The heme ligand (Figure 1.5 ) is a tetradentate ligand.
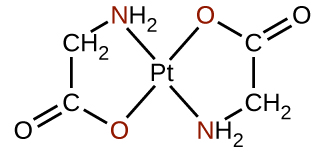
Figure 1.6 : Each of the anionic ligands shown attaches in a bidentate fashion to platinum(II), with both a nitrogen and oxygen atom coordinating to the metal.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















