

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Capillary Zone Electrophoresis
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
22-4-2020
3100
Capillary Zone Electrophoresis
The simplest CE method is capillary zone electrophoresis (CZE), a method by which molecules, ions, or particles are separated solely by their electrophoretic mobility. This technique is based on the idea that the velocity is proportional to the charge to mass ratio. The capillaries are usually made of silica. In uncoated capillaries at pH greater than 3 the SiOH groups are ionized to SiO- (Figure 1.1). The deprotonation creates a negative charge along the capillary wall. This leads to a phenomenon called electroosmotic flow (EOF). The EOF is the overall movement of the sample from the anode to the cathode.
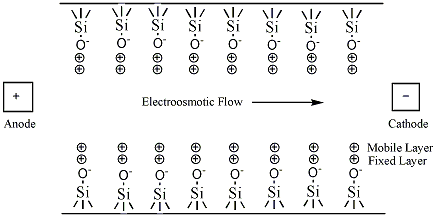
Figure 1.1: SiOH groups being ionized and creating the electroosmotic flow inside of the capillary.
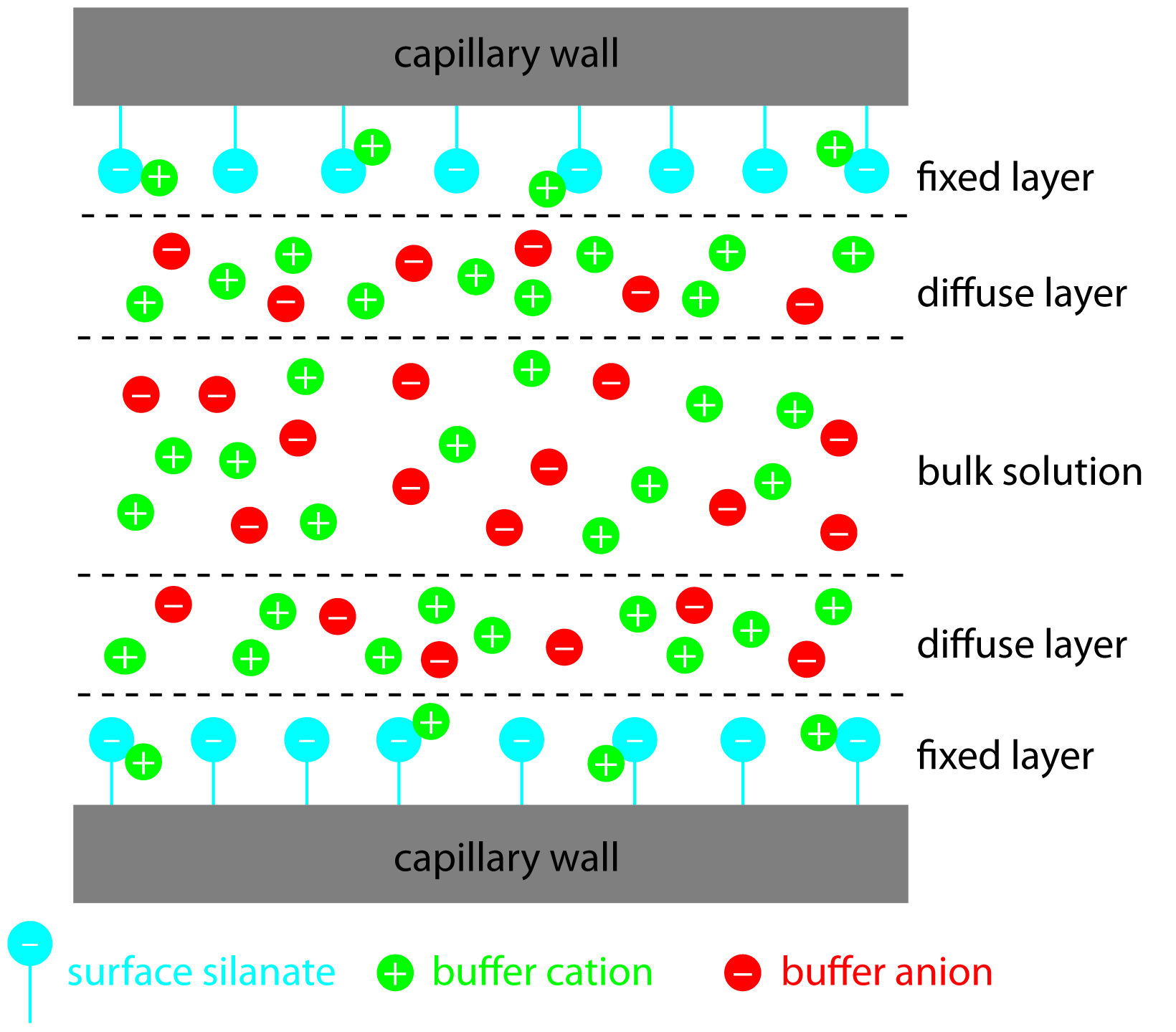
The negative charge on the capillary wall leads to the formation of a double layer of cations along the wall. The inner layer of cations is tightly bound to the capillary wall (cations directly bound to the oxygens of the SiO- group) and the outer layer is a diffuse layer of cations. A zeta potential forms at the boundary between the inner and outer layers of cations. When an electric field is applied, the cations in the diffuse layer move towards the cathode. The cations are more solvated than the anions and pull the bulk solvent towards the cathode. This movement defines the EOF. (Figure 1.2). The relative mobilities of the particles are the same, but now neutral molecules and negative particles are pulled toward the cathode by the EOF.
Figure 1.2: Schematic diagram showing the origin of the double layer within a capillary tube. Although the net charge within the capillary is zero, the distribution of charge is not. The walls of the capillary have an excess of negative charge, which decreases across the fixed layer and the diffuse layer, reaching a value of zero in bulk solution.
While the EOF is affecting all of the components in the sample, the cations have a faster velocity because they are attracted to the cathode, anions are unattracted to the cathode, and neutral molecules are neither attracted or unattracted. Neutral molecules will not be separated from one another. Negatively charged particles will be separated because the electrophoretic mobility counters the EOF. A solute’s total velocity (vtot) as it moves through the capillary is the sum of its electrophoretic velocity and the electroosmotic flow velocity. This concept is demonstrated in Figure 1.3. Cations elute first in an order corresponding to their electrophoretic mobilities, with small, highly charged cations eluting before larger cations of lower charge. Neutral species elute as a single band with an elution rate equal to the electroosmotic flow velocity. Finally, anions are the last components to elute, with smaller, highly charged anions having the longest elution time.
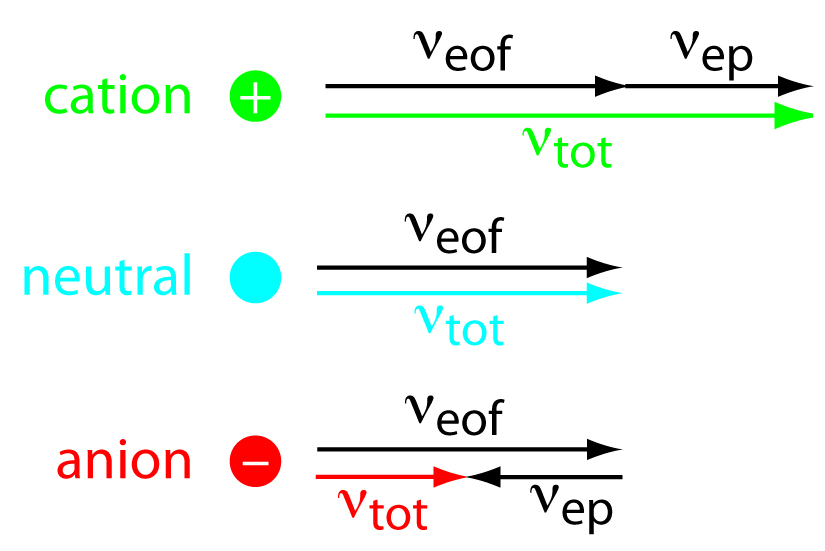
Figure 1.3: Electroosmotic and Electrophoretic flow. A visual explanation for the general elution order in capillary electrophoresis. Each species has the same electroosmotic flow. Cations elute first because they have a positive electrophoretic velocity, νe. Anions elute last because their negative electrophoretic velocity partially offsets the electroosmotic flow velocity. Neutrals elute with a velocity equal to the electroosmotic flow. Figure 1.3 can also be described using the following equations:
The EOF is extremely useful for separating molecules with both positive and negative charges, but the EOF is not necessary. The EOF can be abolished by changing the buffer conditions. Using running buffer at very low pH will abolish the EOF. If low pH is a problem for the stability of the samples, the inside of the capillary can be coated with an uncharged layer. Low concentrations of ionic detergent, below the critical micelle concentration, will also diminish the EOF. There is no separation of molecules with similar charge to mass ratios. It is frequently desirable to improve or alter the separation. Molecules with similar electrophoretic mobilities can be separated by the addition of carrier compounds to the running buffer.
 الاكثر قراءة في التحليل الآلي (الطيفي)
الاكثر قراءة في التحليل الآلي (الطيفي)
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















