
High Performance Liquid Chromatography (2)
 المؤلف:
LibreTexts Project
المؤلف:
LibreTexts Project
 المصدر:
................
المصدر:
................
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 18-4-2020
18-4-2020
 2011
2011
High Performance Liquid Chromatography (2)
Samples collected from medical patients, industry products, and the environment are usually mixtures of many compounds. Often times, doctors, producers, and researchers are interested in specific components in these mixtures, so these mixtures need to be separated. High-performance liquid chromatography (HPLC) offers the ability to do just that. HPLC data can be used to complement gas chromatography (GC) or be an excellent alternative to GC when the components are nonvolatile or would thermodynamically decompose under high temperatures.
In order to separate mixture components, HPLC takes advantages of partitioning between a mobile and stationary phase under a uniform pressure that is typically between 500 to 5000 psi. High pressure is required to obtain a reasonable flow rate through the column. The process begins when a small amount of liquid sample is injected into the column that has a stream of liquid flowing through (which is known as the mobile phase). In partition chromatography, the column is packed with particles that are coated with the stationary phase. The polarity of the component and the type of HPLC being performed determines which phase the component is more attracted to. If the component is more attracted to the mobile phase, it will flow out of the column and have a shorter retention time. If the component is more attracted to the stationary phase, the component will be retained and will, therefore, have a longer retention time. Similar to Capillary Electrophoresis (CE) or Gas Chromatography (GC), these retention times can be used to determine components. Selecting the mobile phase (or solvent) is one of the most important steps when performing HPLC and is selected based on polarity. Solvent polarity relates to the ability of the components to partition into that phase. The polarity scale for different solvents can be found in Table 1.1. These solvents can be used exclusively or mixed to achieve the desired polarity.
Table 1.1: Relative polarities of the mobile phase solvent
| Solvent |
Relative Polarity |
|
Fluoroalkanes
|
<-2
|
|
Cyclohexane
|
.04
|
|
1-Chlorobutane
|
1.0
|
|
Carbon tetrachloride
|
1.6
|
|
Toluene
|
2.4
|
|
Tetrahydrofuran (THF)
|
4.0
|
|
Ethanol
|
4.3
|
|
Methanol
|
5.1
|
|
Acetonitrile
|
5.8
|
|
Ethylene glycol
|
6.9
|
|
Water
|
10.2
|
HPLC can be performed with fixed or variable solvent composition. When the solvent polarity is fixed, it is known as an isocratic run. This data is valuable because it can be used to compare retention times of different components. This method only works when the components have unique retention times under that condition. When the retention times of the components are very similar or extremely different, not every component will be seen in the chromatogram. When solvent polarity is varied throughout the run, this is known as a gradient run. Gradient conditions can be optimized to improve the chance that all the components will be seen on the chromatogram. While all the retention times are not as comparable due to varying conditions, it is useful to see how many components are in the mixture and characterize the components.
During an HPLC analysis of a mixture, the components will separate based on their retention times. This will produce a chromatogram; an example of a chromatogram can be seen in Figure 1.1. Either the peak height or the peak area can be used to estimate the concentration. This can be done because these values are proportional to the concentration when the peaks are sharp, and the flow rate is carefully controlled. A calibration curve can be prepared by plotting either peak height or peak area as a function of concentration.
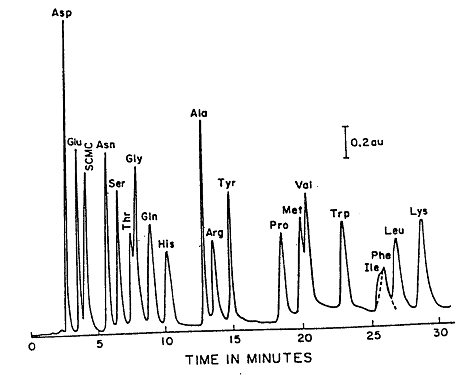
Figure 1.1: Reverse Phase (C18) Separation of Amino Acids.
 الاكثر قراءة في التحليل الآلي (الطيفي)
الاكثر قراءة في التحليل الآلي (الطيفي)
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


