

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Mass Spectrometer : TOF analyzer
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
25-2-2020
1575
Mass Spectrometer : TOF analyzer
Modes
Ions can travel in a linear fashion and be detected by the detector at the opposite end as an ion source. This is called linear TOF. It is different from a reflectron TOF, in which ions are reflected to electrostatic mirror and detected by another detector. Linear TOF spectrum is limited in resolution leading to low mass accuracy. This is because initially different amount of kinetic energy can be attained by the ions with the same charge. This leads to different (m/z) ratio of ions which have different initial velocities. This is partially corrected by reflectron TOF. High energy ions penetrate deeper into the reflectron, taking longer distance and time, while low energy ions do not penetrate as deep into the reflectron and take a shorter path and time. This leads to correction of different times of ions with the same mass and charge. This leads to an increase of resolution to 10,000.
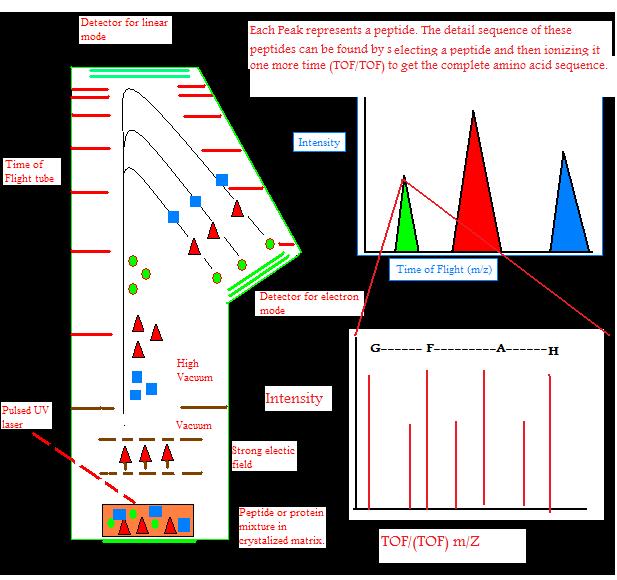
The amount of energy put into ions by the laser initially can also be corrected by a technique called delayed extraction (DE) in which acceleration voltage is applied slightly after the laser pulse. DE TOF increases resolution to 2000.
RE TOF can give full isotopic resolution for molecules up to 15 kDa. However 12C-only ion peak will have very low intensity for molecules bigger than 5kDa. Resolution is further decreased because each ion decays after acceleration and its time of flight can not be adjusted by RE TOF because decay has already occurred. These ions are not detected in RE TOF. However, these ions can be detected in linear TOF, but resolution still decreases because linear TOF has no way of correcting different energy inputs to an ion with the same mass and charge. It is better to use linear mode to get spectra and determine isotopically averaged mass for molecules bigger than 5-10 kDa. Resolution depends on the size of the molecules in a sample. The greater the size that the sample molecule has, the lower the resolution of it.
MALDI-TOF can only be compared to ESI because they are two ways of directly analyzing proteins, peptides, and polymers. MALDI-TOF samples can be reanalyzed while ESI samples can not because ESI is connected to LC column, and the analysis is limited to the width of the chromatographic peak. MALDI-TOF can scan 10 spectras for a peak 10 seconds wide per second, while it takes ESI almost 15 second. MALDI-TOF-MS generates mostly ions +/- 1 charge while ESI generates a charge for every 8-10 amino acids. That means ESI spectra are considerably more complex than MALDI spectra. Coulombic repulsion increases as the charge increase, leading to data deviation, and in MALDI spectra, this repulsion does not occur. However, multiple charges in ESI give better resolution because the higher the mass to charge ratio, the harder it is to get good resolution. In general MALDI is faster than ESI, and enables higher throughput. But ESI is more sensitive.
 الاكثر قراءة في التحليل الآلي (الطيفي)
الاكثر قراءة في التحليل الآلي (الطيفي)
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















