
A good way of picking out the -OH peak
 المؤلف:
..................
المؤلف:
..................
 المصدر:
LibreTexts Project
المصدر:
LibreTexts Project
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 15-9-2019
15-9-2019
 1518
1518
A good way of picking out the -OH peak
If you measure an NMR spectrum for an alcohol like ethanol, and then add a few drops of deuterium oxide, D2O, to the solution, allow it to settle and then re-measure the spectrum, the -OH peak disappears! By comparing the two spectra, you can tell immediately which peak was due to the -OH group.
The reason for the loss of the peak lies in the interaction between the deuterium oxide and the alcohol. All alcohols, such as ethanol, are very, very slightly acidic. The hydrogen on the -OH group transfers to one of the lone pairs on the oxygen of the water molecule. The fact that here we've got "heavy water" makes no difference to that.
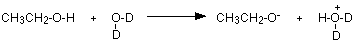
The negative ion formed is most likely to bump into a simple deuterium oxide molecule to regenerate the alcohol - except that now the -OH group has turned into an -OD group.
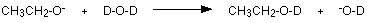
Deuterium atoms don't produce peaks in the same region of an NMR spectrum as ordinary hydrogen atoms, and so the peak disappears. You might wonder what happens to the positive ion in the first equation and the OD- in the second one. These get lost into the normal equilibrium which exists wherever you have water molecules - heavy or otherwise.
2D2O⇌D3O++OH−
 الاكثر قراءة في التشخيص العضوي
الاكثر قراءة في التشخيص العضوي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


