
Reactions of Alkynes : Addition by Electrophilic Reagents
 المؤلف:
..................
المؤلف:
..................
 المصدر:
LibreTexts Project
المصدر:
LibreTexts Project
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 20-7-2019
20-7-2019
 1502
1502
Addition by Electrophilic Reagents
A carbon-carbon triple bond may be located at any unbranched site within a carbon chain or at the end of a chain, in which case it is called terminal. Because of its linear configuration (the bond angle of a sp-hybridized carbon is 180º), a ten-membered carbon ring is the smallest that can accommodate this function without excessive strain. Since the most common chemical transformation of a carbon-carbon double bond is an addition reaction, we might expect the same to be true for carbon-carbon triple bonds. Indeed, most of the alkene addition reactions also take place with alkynes, and with similar regio- and stereoselectivity.
When the addition reactions of electrophilic reagents, such as strong Brønsted acids and halogens, to alkynes are studied we find a curious paradox. The reactions are even more exothermic than the additions to alkenes, and yet the rate of addition to alkynes is slower by a factor of 100 to 1000 than addition to equivalently substituted alkenes. The reaction of one equivalent of bromine with 1-penten-4-yne, for example, gave 4,5-dibromo-1-pentyne as the chief product.
HC≡C-CH2-CH=CH2 + Br2 → HC≡C-CH2-CHBrCH2Br
Although these electrophilic additions to alkynes are sluggish, they do take place and generally display Markovnikov Rule regioselectivity and anti-stereoselectivity. One problem, of course, is that the products of these additions are themselves substituted alkenes and can therefore undergo further addition. Because of their high electronegativity, halogen substituents on a double bond act to reduce its nucleophilicity, and thereby decrease the rate of electrophilic addition reactions. Consequently, there is a delicate balance as to whether the product of an initial addition to an alkyne will suffer further addition to a saturated product. Although the initial alkene products can often be isolated and identified, they are commonly present in mixtures of products and may not be obtained in high yield. The following reactions illustrate many of these features. In the last example, 1,2-diodoethene does not suffer further addition inasmuch as vicinal-diiodoalkanes are relatively unstable.
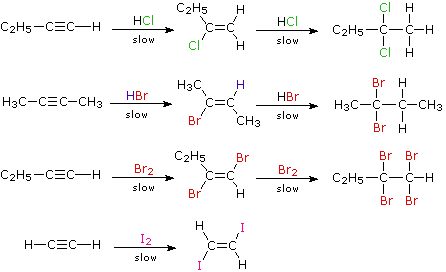
As a rule, electrophilic addition reactions to alkenes and alkynes proceed by initial formation of a pi-complex, in which the electrophile accepts electrons from and becomes weakly bonded to the multiple bond. Such complexes are formed reversibly and may then reorganize to a reactive intermediate in a slower, rate-determining step.
Why are the reactions of alkynes with electrophilic reagents more sluggish than the corresponding reactions of alkenes? After all, addition reactions to alkynes are generally more exothermic than additions to alkenes, and there would seem to be a higher π-electron density about the triple bond ( two π-bonds versus one ). Two factors are significant in explaining this apparent paradox. First, although there are more π-electrons associated with the triple bond, the sp-hybridized carbons exert a strong attraction for these π-electrons, which are consequently bound more tightly to the functional group than are the π-electrons of a double bond. This is seen in the ionization potentials of ethylene and acetylene.
| Acetylene |
HC≡CH + Energy → [HC≡CH •(+) + e(–) |
|
ΔH = +264 kcal/mole |
| Ethylene |
H2C=CH2 + Energy → [H2C=CH2] •(+) + e(–) |
|
ΔH = +244 kcal/mole |
| Ethane |
H3C–CH3 + Energy → [H3C–CH3] •(+) + e(–) |
|
ΔH = +296 kcal/mole |
As defined by the preceding equations, an ionization potential is the minimum energy required to remove an electron from a molecule of a compound. Since pi-electrons are less tightly held than sigma-electrons, we expect the ionization potentials of ethylene and acetylene to be lower than that of ethane, as is the case. Gas-phase proton affinities show the same order, with ethylene being more basic than acetylene, and ethane being less basic than either. Since the initial interaction between an electrophile and an alkene or alkyne is the formation of a pi-complex, in which the electrophile accepts electrons from and becomes weakly bonded to the multiple bond, the relatively slower reactions of alkynes becomes understandable.
A second factor is presumed to be the stability of the carbocation intermediate generated by sigma-bonding of a proton or other electrophile to one of the triple bond carbon atoms. This intermediate has its positive charge localized on an unsaturated carbon, and such vinyl cations are less stable than their saturated analogs. Indeed, we can modify our earlier ordering of carbocation stability to include these vinyl cations in the manner shown below. It is possible that vinyl cations stabilized by conjugation with an aryl substituent are intermediates in HX addition to alkynes of the type Ar-C≡C-R, but such intermediates are not formed in all alkyne addition reactions.
Carbocation
Stability |
CH3(+) |
≈ |
RCH=CH(+) |
< |
RCH2(+) |
≈ |
RCH=CR(+) |
< |
R2CH(+) |
≈ |
CH2=CH-CH2(+) |
< |
C6H5CH2(+) |
≈ |
R3C(+) |
| Methyl |
|
1°-Vinyl |
|
1° |
|
2°-Vinyl |
|
2° |
|
1°-Allyl |
|
1°-Benzyl |
|
3° |
|
Application of the Hammond postulate indicates that the activation energy for the generation of a vinyl cation intermediate would be higher than that for a lower energy intermediate. This is illustrated for alkenes versus alkynes by the following energy diagrams.
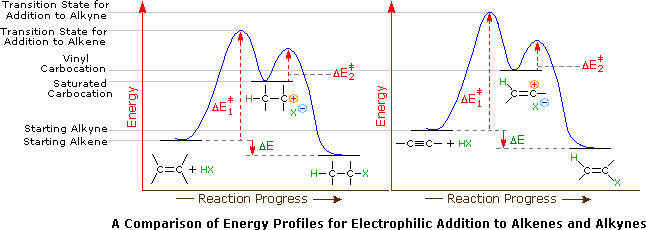
Despite these differences, electrophilic additions to alkynes have emerged as exceptionally useful synthetic transforms.
 الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


