
Representations of Molecular Structures
 المؤلف:
..................
المؤلف:
..................
 المصدر:
LibreTexts Project
المصدر:
LibreTexts Project
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 26-5-2019
26-5-2019
 3796
3796
Representations of Molecular Structures
Molecular formulas give only the elemental composition of molecules. In contrast, structural formulas show which atoms are bonded to one another and, in some cases, the approximate arrangement of the atoms in space. Knowing the structural formula of a compound enables chemists to create a three-dimensional model, which provides information about how that compound will behave physically and chemically.
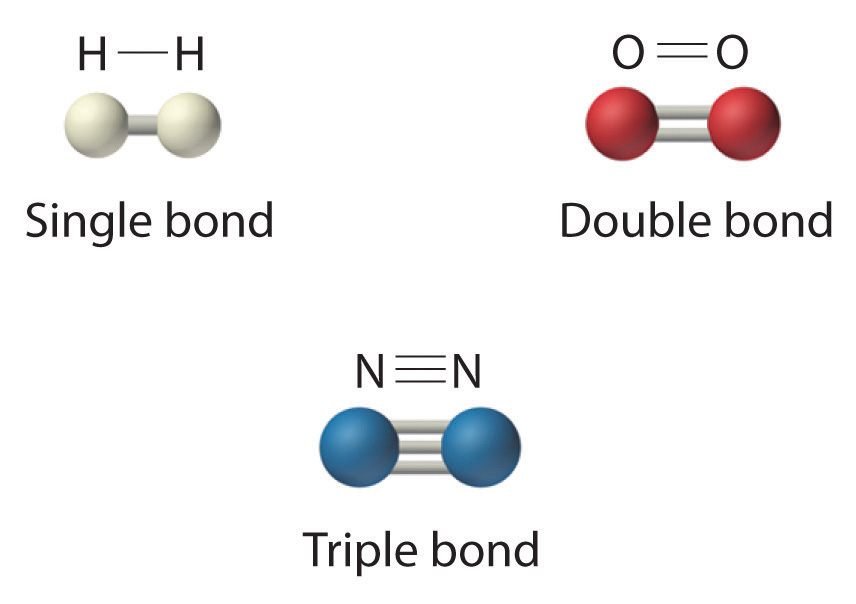
Figure 1.1
: Molecules That Contain Single, Double, and Triple Bonds. Hydrogen (H2) has a single bond between atoms. Oxygen (O2) has a double bond between atoms, indicated by two lines (=). Nitrogen (N2) has a triple bond between atoms, indicated by three lines (≡). Each bond represents an electron pair.
The structural formula for H2 can be drawn as H–H and that for I2 as I–I, where the line indicates a single pair of shared electrons, a single bond. Two pairs of electrons are shared in a double bond, which is indicated by two lines—for example, O2 is O=O. Three electron pairs are shared in a triple bond, which is indicated by three lines—for example, N2 is N≡N (see Figure 1.1). Carbon is unique in the extent to which it forms single, double, and triple bonds to itself and other elements. The number of bonds formed by an atom in its covalent compounds is not arbitrary. Hydrogen, oxygen, nitrogen, and carbon have very strong tendencies to form substances in which they have one, two, three, and four bonds to other atoms, respectively (Table 1.1).
Table 1.1 : The Number of Bonds That Selected Atoms Commonly Form to Other Atoms
| Atom |
Number of Bonds |
| H (group 1) |
1 |
| O (group 16) |
2 |
| N (group 15) |
3 |
| C (group 14) |
4 |
The structural formula for water can be drawn as follows:
Because the latter approximates the experimentally determined shape of the water molecule, it is more informative. Similarly, ammonia (NH3) and methane (CH4) are often written as planar molecules:
As shown in Figure 1.2 , however, the actual three-dimensional structure of NH3 looks like a pyramid with a triangular base of three hydrogen atoms. The structure of CH4, with four hydrogen atoms arranged around a central carbon atom as shown in Figure 1.2
, is tetrahedral: the hydrogen atoms are positioned at every other vertex of a cube. Many compounds—carbon compounds, in particular—have four bonded atoms arranged around a central atom to form a tetrahedron.
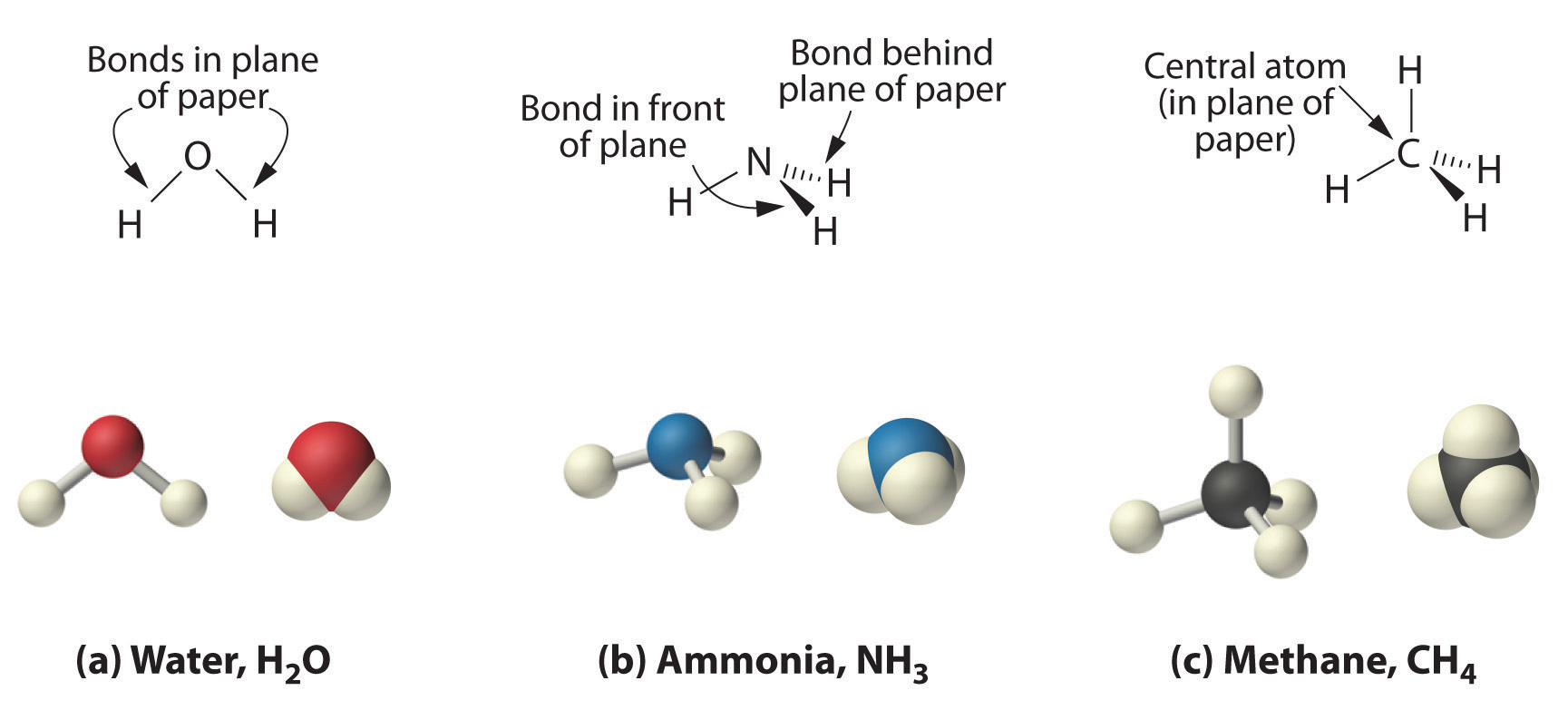
Figure 1.2
: The Three-Dimensional Structures of Water, Ammonia, and Methane. (a) Water is a V-shaped molecule, in which all three atoms lie in a plane. (b) In contrast, ammonia has a pyramidal structure, in which the three hydrogen atoms form the base of the pyramid and the nitrogen atom is at the vertex. (c) The four hydrogen atoms of methane form a tetrahedron; the carbon atom lies in the center.
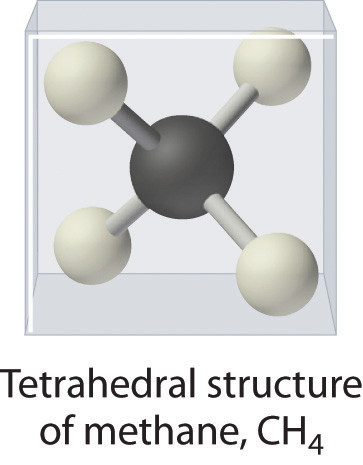
Methane CH4 has a three-dimensional, tetrahedral structure.
Figures 1.1 , 1.1, and 1.2 illustrate different ways to represent the structures of molecules. It should be clear that there is no single “best” way to draw the structure of a molecule; the method used depends on which aspect of the structure should be emphasized and how much time and effort is required. Figure 1.3 shows some of the different ways to portray the structure of a slightly more complex molecule: methanol. These representations differ greatly in their information content. For example, the molecular formula for methanol (Figure 1.3a) gives only the number of each kind of atom; writing methanol as CH4O tells nothing about its structure. In contrast, the structural formula (Figure 1.3b) indicates how the atoms are connected, but it makes methanol look as if it is planar (which it is not). Both the ball-and-stick model (part (c) in Figure 1.3) and the perspective drawing (Figure 1.3d) show the three-dimensional structure of the molecule. The latter (also called a wedge-and-dash representation) is the easiest way to sketch the structure of a molecule in three dimensions. It shows which atoms are above and below the plane of the paper by using wedges and dashes, respectively; the central atom is always assumed to be in the plane of the paper. The space-filling model (part (e) in Figure 1.3) illustrates the approximate relative sizes of the atoms in the molecule, but it does not show the bonds between the atoms. In addition, in a space-filling model, atoms at the “front” of the molecule may obscure atoms at the “back.”
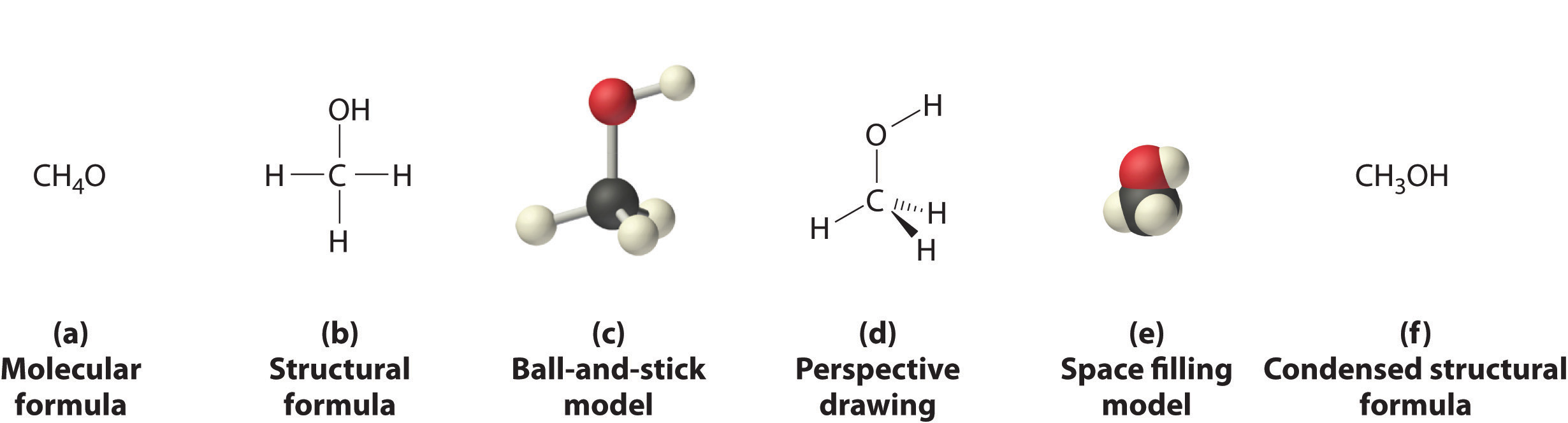
Figure 1.3 : Different Ways of Representing the Structure of a Molecule. (a) The molecular formula for methanol gives only the number of each kind of atom present. (b) The structural formula shows which atoms are connected. (c) The ball-and-stick model shows the atoms as spheres and the bonds as sticks. (d) A perspective drawing (also called a wedge-and-dash representation) attempts to show the three-dimensional structure of the molecule. (e) The space-filling model shows the atoms in the molecule but not the bonds. (f) The condensed structural formula is by far the easiest and most common way to represent a molecule.
Although a structural formula, a ball-and-stick model, a perspective drawing, and a space-filling model provide a significant amount of information about the structure of a molecule, each requires time and effort. Consequently, chemists often use a condensed structural formula (part (f) in Figure 1.3 ), which omits the lines representing bonds between atoms and simply lists the atoms bonded to a given atom next to it. Multiple groups attached to the same atom are shown in parentheses, followed by a subscript that indicates the number of such groups. For example, the condensed structural formula for methanol is CH3OH, which indicates that the molecule contains a CH3 unit that looks like a fragment of methane (CH4). Methanol can therefore be viewed either as a methane molecule in which one hydrogen atom has been replaced by an –OH group or as a water molecule in which one hydrogen atom has been replaced by a –CH3 fragment. Because of their ease of use and information content, we use condensed structural formulas for molecules throughout this text. Ball-and-stick models are used when needed to illustrate the three-dimensional structure of molecules, and space-filling models are used only when it is necessary to visualize the relative sizes of atoms or molecules to understand an important point.
 الاكثر قراءة في كيمياء عامة
الاكثر قراءة في كيمياء عامة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


