

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Nitrogen Compounds
المؤلف:
........
المصدر:
LibreTexts Project
الجزء والصفحة:
............
19-10-2018
1903
Nitrogen Compounds
The two most common compounds of nitrogen are Potassium Nitrate (KNO3) and Sodium Nitrate (NaNO3). These two compounds are formed by decomposing organic matter that has potassium or sodium present and are often found in fertilizers and byproducts of industrial waste. Most nitrogen compounds have a positive Gibbs free energy (i.e., reactions are not spontaneous).
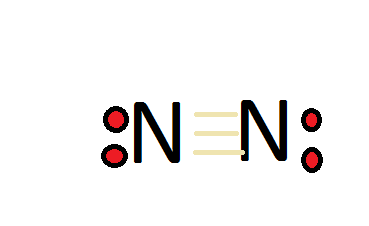
Figure 1.1
: Lewis Dot structure of molecular nitrogen
The dinitrogen molecule (N2) is an "unusually stable" compound, particularly because nitrogen forms a triple bond with itself. This triple bond is difficult hard to break. For dinitrogen to follow the octet rule, it must have a triple bond. Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have a triple bond. The compound is also very inert, since it has a triple bond. Triple bonds are very hard to break, so they keep their full valence shell instead of reacting with other compounds or atoms. Think of it this way, each triple bond is like a rubber band, with three rubber bands, the nitrogen atoms are very attracted to each other.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















