

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
RATE LAWS
المؤلف:
Arun Bahl, B.S. Bahl and G.D.Tuli
المصدر:
Essential Physical Chemistry
الجزء والصفحة:
p 734
22-9-2018
688
RATE LAWS
At a fixed temperature the rate of a given reaction depends on concentration of reactants.
The exact relation between concentration and rate is determined by measuring the reaction rate with different initial reactant concentrations. By a study of numerous reactions it is shown that : the rate of a reaction is directly proportional to the reactant concentrations, each concentration being raised to some power.
Thus for a substance A undergoing reaction,
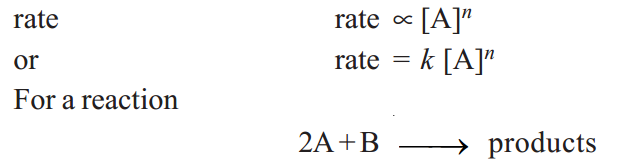 …..(1)
…..(1)
the reaction rate with respect to Aor Bis determined by varying the concentration of one reactant, keeping that of the other constant. Thus the rate of reaction may be expressed as
rate = k [A]m[B]n ……….…..(2)
Expressions such as (1) and (2) tell the relation between the rate of a reaction and reactant concentrations.
An expression which shows how the reaction rate is related to concentrations is called the rate law or rate equation.
The power (exponent) of concentration nor min the rate law is usually a small whole number integer (1, 2, 3) or fractional. The proportionality constant kis called the rate constantfor the reaction.
Examples of rate law :
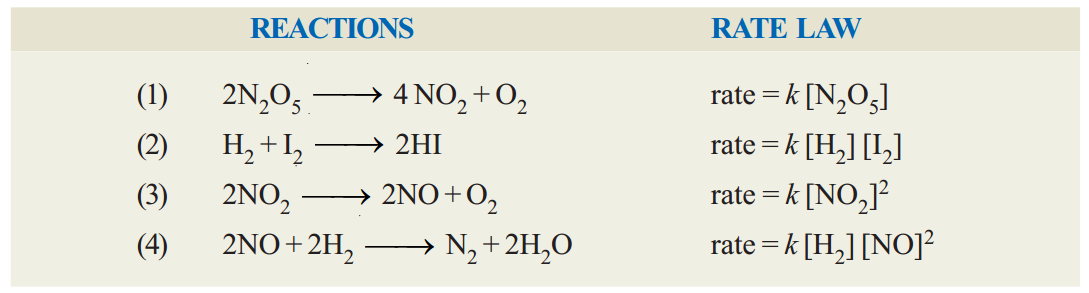
In these rate laws where the quotient or concentration is not shown, it is understood to be 1. That is [H2]1= [H2].
It is apparent that the rate law for a reaction must be determined by experiment. It cannot be written by merely looking at the equation with a background of our knowledge of Law of Mass Action.
However, for some elementary reactions the powers in the rate law may correspond to coefficients in the chemical equation. But usually the powers of concentration in the rate law are different from coefficients. Thus for the reaction (4) above, the rate is found to be proportional to [H2] although thequotient of H2 in the equation is 2. For NO the rate is proportional to [NO]2 and power ‘2’ corresponds to the coefficient.
 الاكثر قراءة في حركية التفاعلات الكيميائية
الاكثر قراءة في حركية التفاعلات الكيميائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















