
The Beckmann Rearrangement
 المؤلف:
William Reusch
المؤلف:
William Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 17-9-2018
17-9-2018
 3080
3080
The Beckmann Rearrangement
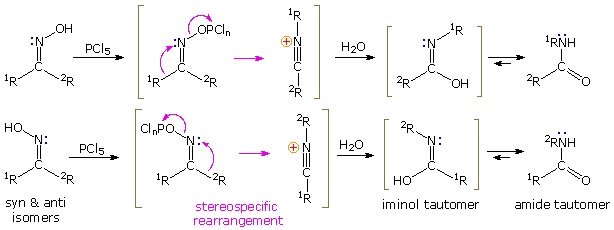
If the hydroxyl group of a ketoxime is removed by the action of strong acid or phosphorous pentachloride, followed by hydrolysis, an amide is formed. Complete removal of the derivatized hydroxyl group and its bonding electron pair would generate a divalent sp-hybridized azacation of the type depicted in the previous diagram. Were this to occur, both carbon substituents (1R & 2R) would be candidates for the subsequent 1,2-shift. In practice, however, it is always the group anti to the departing OH that migrates to nitrogen. This stereospecificity indicates that the 1,2-shift is concerted with N-O cleavage, as shown below. The resulting N-alkylated nitrilium intermediate will react with nucleophiles (e.g. water) at the electrophilic carbon atom adjacent to the "onium" nitrogen. Note that the structure drawn for this intermediate is the more favored of two resonance contributors, inasmuch as all heavy atoms have filled valence shell octets. Bonding of a nucleophile to the nitrogen atom would require expanding its valence shell to include ten electrons, or formation of an unstable dipolar species. The initial product from hydration at carbon is an iminol, which immediately tautomerizes to the more stable amide. Reactions with PCl5 probably give an iminochloride, and this in turn is hydrolyzed to the same amide.
The first example in the following group of reactions is a typical Beckmann rearrangement. The oxime from cyclohexanone has identical carbonyl substituents. and therefore exists as a single isomer. The product of the rearrangement is a lactam (a cyclic amide), which can be hydrolyzed to an omega-amino acid. This lactam serves as an important industrial precursor to nylon 6. The second example involves an oxime derivative with different carbonyl substituents, which exists as a pair of stereoisomers (syn & anti). The anti isomer rearranges by a 1,2-phenyl shift, whereas the syn isomer undergoes a 1,2-isopropyl shift. The former reaction is much faster than the latter, presumably because it proceeds by way of a relatively stable phenonium ion intermediate (structure in shaded box). Note that the picrate leaving group (2,4,6-trinitrophenolate) is a stable anion. Example #3 is another case that demonstrates the stereospecificity of the Beckmann rearrangement. The 1,2-shift of the ortho-phenol substituent is faster than that of the unsubstituted phenyl group, and the hydroxyl is ideally located to bond to the electrophilic carbon of the intermediate. Consequently, the product from the anti isomer is a benzoxazole heterocycle.
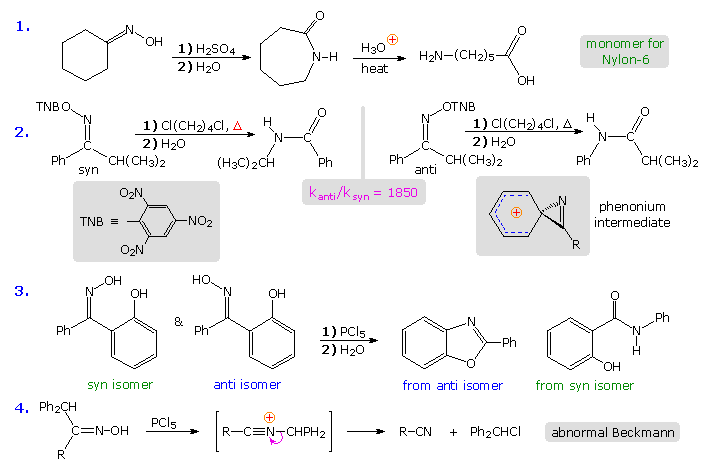
The fourth example above shows an unusual divergence in behavior that sometimes occurs when the migrating substituent fragments from the intermediate, leaving a nitrile as a stable product. This has been called an abnormal Beckmann reaction.
The rigid configuration of the phenonium cation shown above imposes a structural constraint that is nicely demonstrated by the rates of rearrangement of some fused ring bicyclic compounds.
Because of the carbon chain joining the oxime function to the ortho-carbon of the benzene ring, the phenonium ion that normally facilitates phenyl migration may be unable to assume its preferred structure (three-membered ring orthogonal to the phenyl ring). The three-carbon bridging chain for n = 6 is such a case, and rearrangement of the anti isomer is very slow. As the length of the bridging chain increases, its constraint is less severe, and the rate of rearrangement increases. The eight-membered oxime picrate hydrolyzes rapidly, producing a nine-membered lactam in high yield.
|
R2C=N-NH2 + HNO2  RCONHR + N2 RCONHR + N2
|
Beckmann type rearrangements may also be carried out by treating hydrazones with nitrous acid, as shown on the right. As a rule, this is a less desirable procedure because pure hydrazones are more difficult to acquire, and the yield of pure product is inferior.
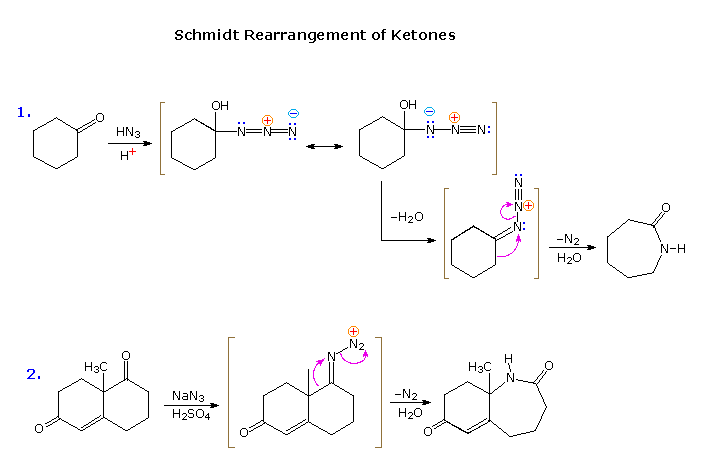
A direct rearrangement of ketones, thereby avoiding the necessity of preparing an derivative, is possible by a procedure known as the Schmidt rearrangement. Acid-catalyzed addition of hydrazoic acid to the carbonyl group of a ketone creates an unstable azidocarbinol that, on dehydration, produces the same triazonium cation presumably formed as an intermediate in the nitrous acid deamination of a hydrazone. Rearrangement of this species by rapid nitrogen loss then initiates a Beckmann-like rearrangement. By clicking the upper diagram a second time, two examples of the Schmidt rearrangement will be presented.
 الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


