

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Radical Recombination Reactions
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
28-8-2018
3799
Radical Recombination Reactions
Radical coupling (recombination) reactions are very fast, having activation energies near zero. Such reactions were noted earlier in the context of the cage effect. The only reason radical coupling reactions do not dominate free radical chemistry is that most radicals have very short lifetimes and are present in very low concentration. Consequently, if short lived radicals are to contribute to useful synthetic procedures by way of a radical coupling, all the events leading up to the coupling must take place in a solvent cage.
To this end, a clever reaction sequence, resulting in intramolecular functionalization of an isolated methyl group, was designed by Derek Barton, and has been named the "Barton reaction". A general outline of this procedure is shown below. A pair of radicals is generated by photohomolysis of the weak N–O bond of a nitrite ester. The oxy radical abstracts a hydrogen atom from a nearby carbon, and the resulting radical couples with •NO to give a nitroso compound. Subsequent tautomerism of the nitroso product, followed by hydrolysis, converts this function to a carbonyl group. If oxygen is present, it bonds to the carbon radical before the •NO coupling occurs. This eventually leads to a nitrate ester on the newly functionalized carbon.
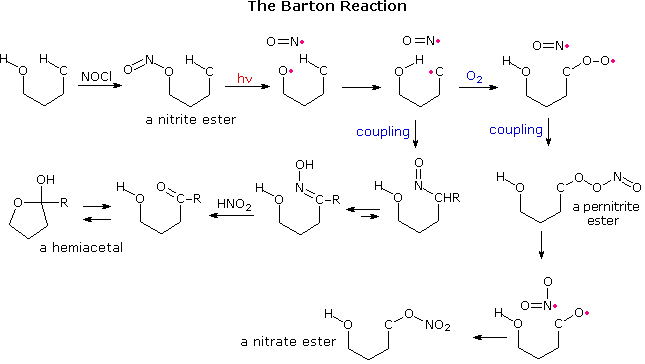
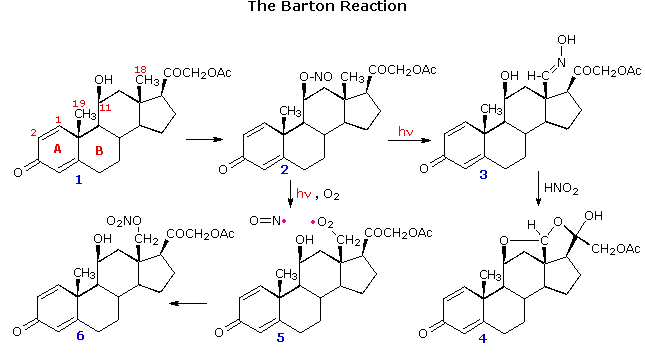
For the Barton reaction to work, it is necessary that the initial oxy radical be firmly oriented close to the C–H hydrogen. This requirement is satisfied by appropriate steroid substrates, as demonstrated above by clicking on the diagram. The nitrite ester of an axial oriented 11-hydroxyl group is prepared by reaction with nitrosyl chloride, 2. Photolysis generates an oxy radical that is located close to the 18-methyl group. Hydrogen abstraction, followed by coupling of the CH2• to •NO gave a nitroso compound that tautomerizes to an oxime, 3. Hydrolysis of 3 by nitrous acid yields the corresponding aldehyde, which immediately forms the bis-hemiacetal, 4. In the presence of oxygen, the corresponding nitrate ester, 6, is produced.
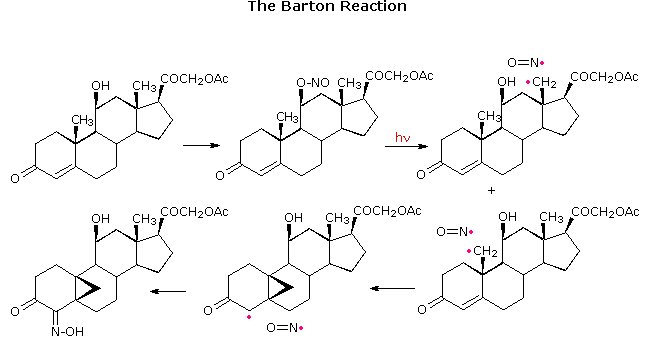
From models it appears that the axial-11-hydroxyl group is equally close to both the 18- and 19-methyl groups. However, the two double bonds in ring A slightly change the orientation of the 19-methyl group, and hydrogen atom abstraction from that location does not occur. If the double bond between carbons 1 & 2 is removed the orientation of the the 19-methyl group improves, and hydrogen atom abstraction from both methyl sites takes place. Note that the 1º-radical at C-19 adds to the double bond before coupling with •NO takes place. All these radical reactions must occur rapidly in a solvent cage before the •NO escapes.
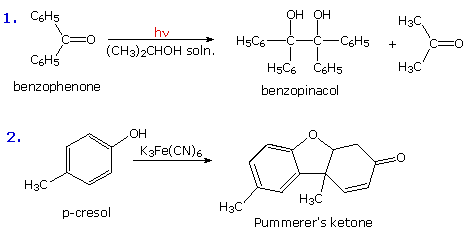
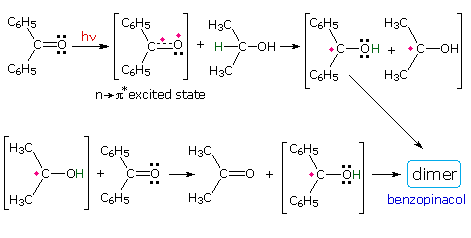
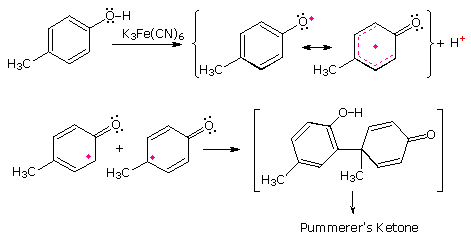
Stabilized free radicals have sufficiently long lifetimes to permit coupling outside solvent cage confinement. The following diagram shows two such coupling reactions. The first is the photochemical reduction of benzophenone to benzopinacol. The second is an example of the oxidative coupling of phenols, a transformation that is an important step in the biosynthesis of alkaloids.
 الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















