

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
carbonates and hydrogencarbonates of group 1 metals
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 265
15-1-2018
1482
carbonates and hydrogencarbonates of group 1 metals
The properties of alkali metal salts of most oxoacids depend on the oxoanion present and not onm the cation; thus we tend to discuss salts of oxoacids under the appropriate acid. However, we single out the carbonates and hydrogencarbonates because of their importance. Whereas Li2CO3 is sparingly soluble in water, the remaining carbonates of the group 1 metals are very soluble.
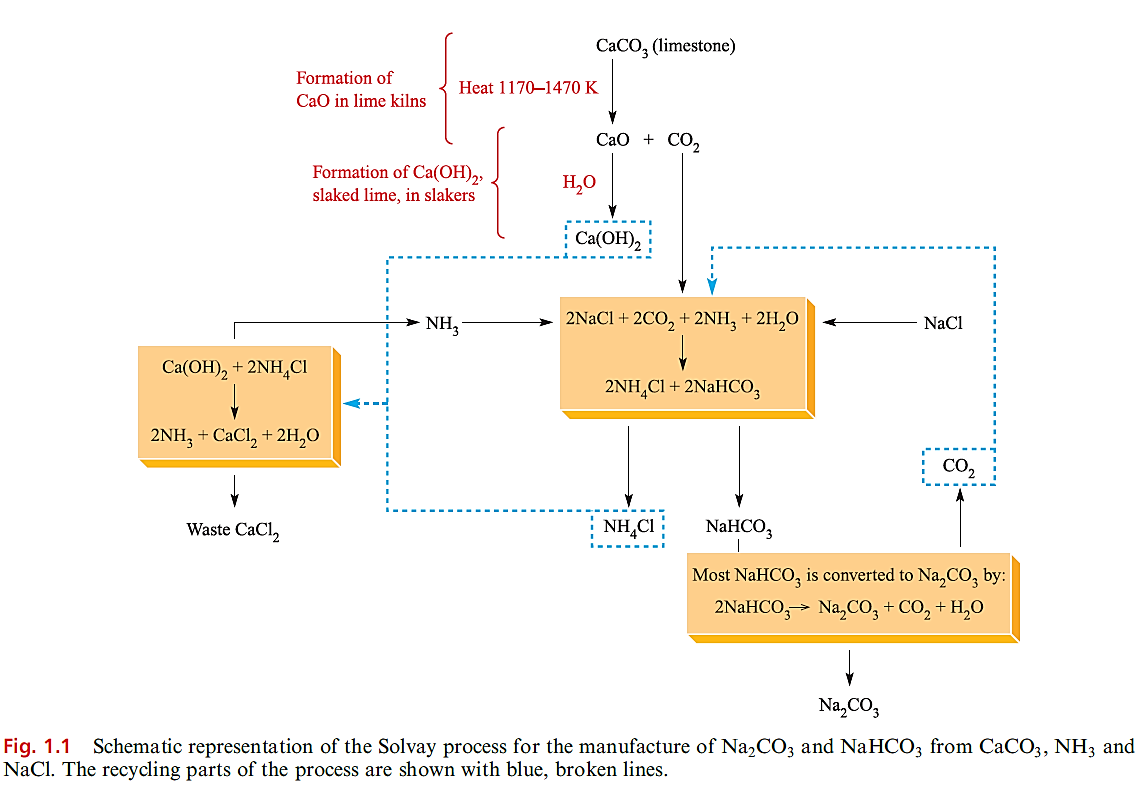
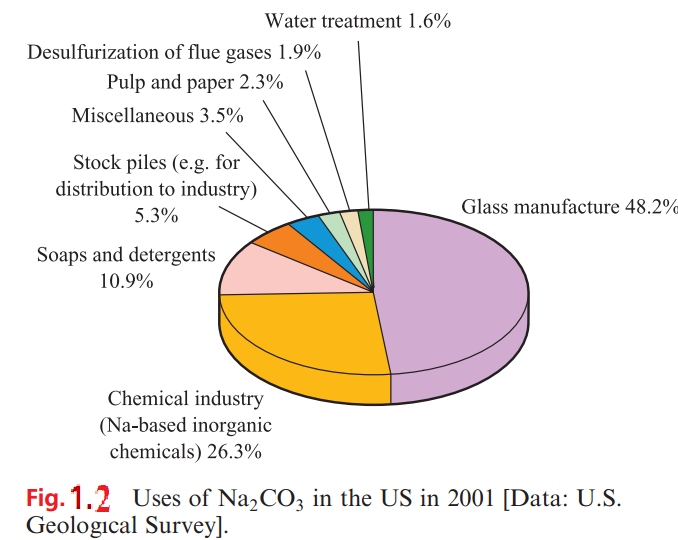
In many countries, sodium carbonate (soda ash) and sodium hydrogencarbonate (commonly called sodium bicarbonate) are manufactured by the Solvay process (Figure 1.1), but this is being superseded where natural sources of the mineral trona, Na2 CO3.NaHCO3.2H2O, are available. Figure 1.1 shows that NH3 can be recycled, but most waste CaCl2 is dumped (e.g. into the sea) or used in winter road clearance. In 2001, _35 Mt of sodium carbonate was produced worldwide, 10.3Mt in the US. The US (a net exporter of Na2CO3) consumed _6.4 Mt of sodium carbonate in 2003; uses are summarized in Figure 1.2.
Sodium hydrogencarbonate, although a direct product in the Solvay process, is also manufactured by passing CO2 through aqueous Na2CO3 or by dissolving trona in H2O saturated with CO2. Its uses include those as a foaming agent, a food additive (e.g. baking powder) and an effervescent in pharmaceutical products. The Solvay company has now developed a process for using NaHCO3 in pollution control, e.g. by neutralizing SO2 or HCl in industrial and other waste emissions.
There are some notable differences between Na and other alkali metal [CO3]2- and [HCO3]- salts. Whereas NaHCO3 can be separated in the Solvay process by precipitation, the same is not true of KHCO3. Hence, K2CO3 is produced, not via KHCO3, but by the reaction of KOH with CO2; K2CO3 has uses in the manufacture of certain glasses and ceramics. Among its applications, KHCO3 is used as a buffering agent in water treatment and wine production. Lithium carbonate is only sparingly soluble in water; ‘LiHCO3’ has not been isolated. The thermal stabilities of the group 1 metal carbonates with increase down the group as rM+ increases, lattice energy being a crucial factor. Such a trend in stability is common to all series of oxo-salts of the alkali metals.
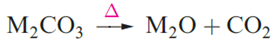
The solid state structures of NaHCO3 and KHCO3 exhibit hydrogen bonding . In KHCO3, the anions associate in pairs whereas in NaHCO3, infinite chains are present. In each case, the hydrogen bonds are asymmetrical. Sodium silicates are also of great commercial importance:
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















