
Accuracy
 المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
 المصدر:
Fundamentals of Analytical Chemistry
المصدر:
Fundamentals of Analytical Chemistry
 الجزء والصفحة:
9th ed - p85
الجزء والصفحة:
9th ed - p85
 24-8-2016
24-8-2016
 3194
3194
Accuracy
Accuracy indicates the closeness of the measurement to the true or accepted value and is expressed by the error. Figure 1-1 illustrates the difference between accuracy and precision. Note that accuracy measures agreement between a result and the accepted value. Precision, on the other hand, describes the agreement among several results obtained in the same way. We can determine precision just by measuring replicate samples. Accuracy is often more difficult to determine because the true value is usually unknown. An accepted value must be used instead. Accuracy is expressed in terms of either absolute or relative error.
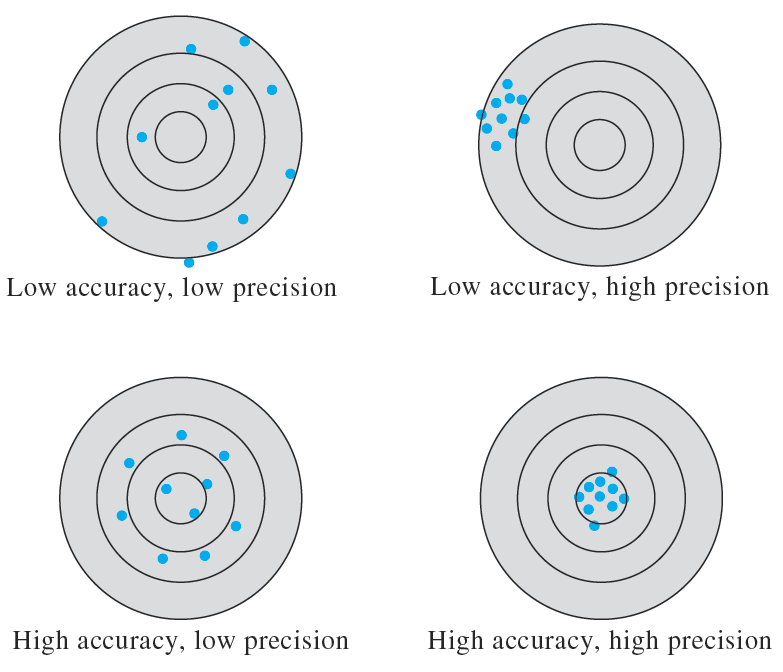
Figure 1-1 Illustration of accuracy and precision using the pattern of darts on a dartboard. Note that we can have very precise results (upper right) with a mean that is not accurate and an accurate mean (lower left) with data points that are imprecise.
 الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


